Abstract
Mutually beneficial associations between plants and soil fungi, mycorrhizas, are one of the most important terrestrial symbioses. These partnerships are thought to have propelled plant terrestrialisation some 500 million years ago and today they play major roles in ecosystem functioning. It has long been known that bryophytes harbour, in their living tissues, fungal symbionts, recently identified as belonging to the three mycorrhizal fungal lineages Glomeromycotina, Ascomycota and Basidiomycota. Latest advances in understanding of fungal associations in bryophytes have been largely driven by the discovery, nearly a decade ago, that early divergent liverwort clades, including the most basal Haplomitriopsida, and some hornworts, engage with a wider repertoire of fungal symbionts than previously thought, including endogonaceous members of the ancient sub-phylum Mucoromycotina. Subsequent global molecular and cytological studies have revealed that Mucoromycotina symbionts, alongside Glomeromycotina, are widespread in both complex and simple thalloid liverworts and throughout hornworts, with physiological studies confirming that, in liverworts at least, these associations are mycorrhizal-like, and highlighting important functional differences between Mucoromycotina and Glomeromycotina symbioses. Whether a more prominent role of Mucoromycotina symbionts in plant nitrogen nutrition, as identified in liverworts, extends to other plant lineages, including the flowering plants, is a major topic for future research.
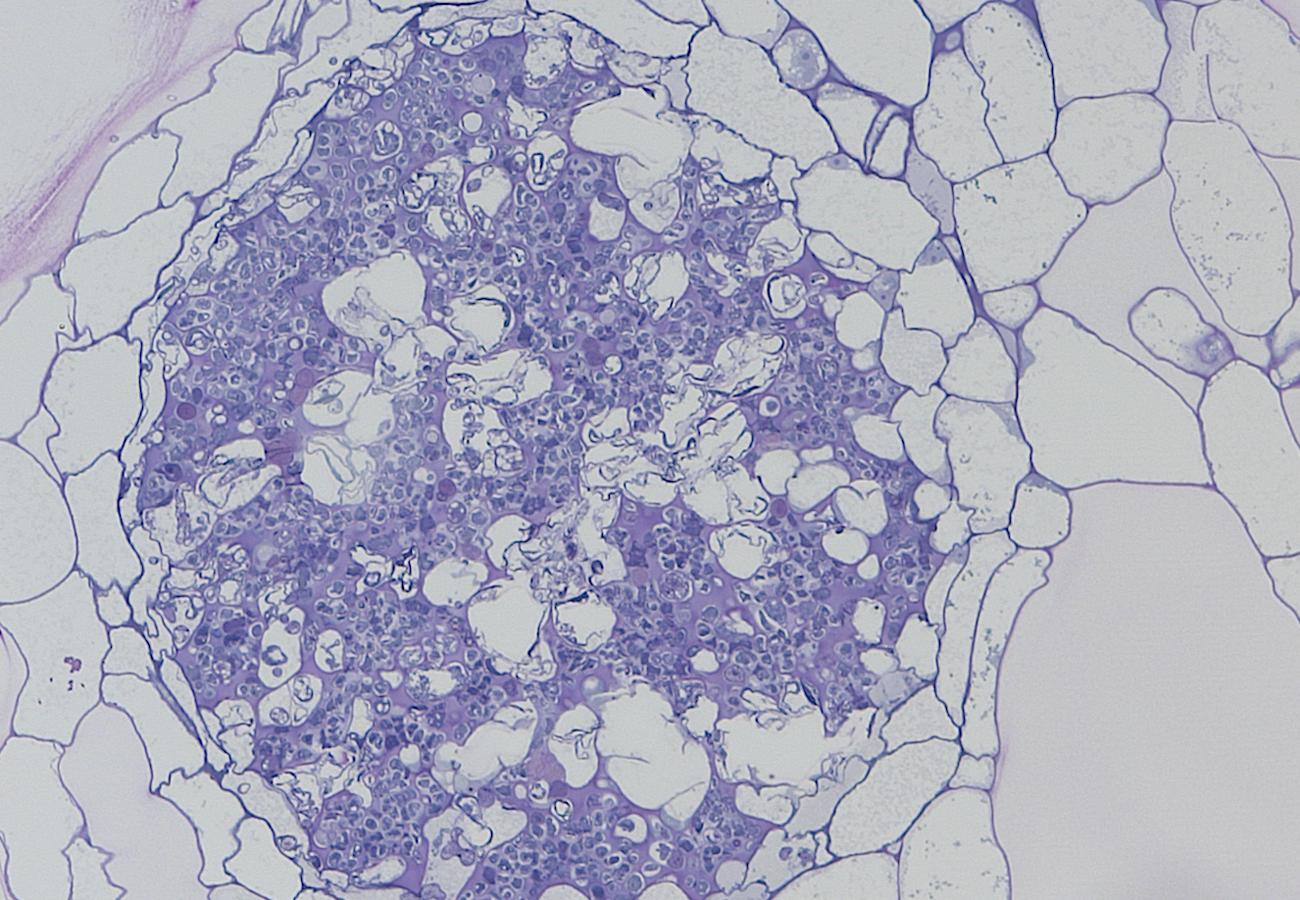
The latest finding that ascomycete symbionts of leafy liverworts are not restricted to one fungus, Rhizoscyphus ericae, but include species in the genus Meliniomyces, as shown here in Mylia anomala, together with the recent demonstration that R. ericae forms nutritional mutualisms with the rhizoids of Cephalozia bicuspidata, fill other major gaps in our growing knowledge of fungal associations across land plants.
Downloads
References
Adams, D.G. (2002) Cyanobacteria in symbiosis with hornworts and liverworts. In: Whitton, B.A. & Potts, M. (Eds.) Ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht, pp. 523–561. https://doi.org/10.1007/0-306-48005-0_7
Adams, D.G. & Duggan, P.S. (2008) Cyanobacteria-bryophyte symbioses. Journal of Experimental Botany 59: 1047–1058. https://doi.org/10.1093/jxb/ern005
Azcón, R., Rubio, R. & Barea, J.M. (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytologist 117: 339–404. https://doi.org/10.1111/j.1469-8137.1991.tb00003.x
Berbee, M., James, T. & Strullu-Derrien, C. (2017) Early diverging fungi: Diversity and impact at the dawn of terrestrial life. Annual Review of Microbiology 71: 41–59. https://doi.org/10.1146/annurev-micro-030117-020324
Bergman, B., Johansson, C. & Soderback E. (1992) The Nostoc–Gunnera symbiosis. New Phytologist 122: 379–400. https://doi.org/10.1111/j.1469-8137.1992.tb00067.x
Bidartondo, M.I., Bruns, T.D., Weiss, M., Sérgio, C. & Read, D.J. (2003) Specialized cheating of the ectomycorrhizal symbiosis by an epiparasitic liverwort. Proceedings of the Royal Society B 270: 835–842. https://doi.org/10.1098/rspb.2002.2299
Bidartondo, M.I. & Duckett, J.G. (2010) Conservative ecological and evolutionary patterns in liverwort-fungal symbioses. Proceedings of the Royal Society B 277: 485–492. https://doi.org/10.1098/rspb.2009.1458
Bidartondo, M.I., Read, D.J., Trappe, J.M., Merckx, V., Ligrone, R. & Duckett, J.G. (2011) The dawn of symbiosis between plants and fungi. Biology Letters 7: 574–577. https://doi.org/10.1098/rsbl.2010.1203
Bonfante, P. & Venice, F. (2020) Mucoromycota: going to the roots of plant-interacting fungi. Fungal Biology Reviews 34: 100–113. https://doi.org/10.1016/j.fbr.2019.12.003
Bowman, J.L., Araki, T., Arteaga-Vazquez, M.A., Berger, F., Dolan, L., Hasel-off, J., Ishizaki, K., Kyozuka, J., Lin, S.S., Nagasaki, H., Nakagami, H., Nakajima, K., Nakamura, Y., Ohashi-Ito, K., Sawa, S., Shimamura, M., Solano, R., Tsukaya, H., Ueda, T., Watanabe, Y., Yamato, K.T., Zachgo, S. & Kohchi, T. (2016) The naming of names: guidelines for gene nomenclature in Marchantia. Plant Cell Physiology 57: 257–261. https://doi.org/10.1093/pcp/pcv193
Bowman, J.L., Kohchi, T., Yamato, K.T., Jenkins, J., Shu, S., Ishizaki, K., Yamaoka, S., Nishihama, R., Nakamura, Y., Berger, F., Adam, C., Aki, S.S., Althoff, F., Araki, T., Arteaga-Vazquez, M.A., Balasubrmanian, S., Barry, K., Bauer, D., Boehm, C.R., Briginshaw, L., Caballero-Perez, J., Catarino, B., Chen, F., Chiyoda, S., Chovatia, M., Davies, K.M., Delmans, M., Demura, T., Dierschke, T., Dolan, L., Dorantes-Acosta, A.E., Eklund, D.M., Florent, S.N., Flores-Sandoval, E., Fujiyama, A., Fukuzawa, H., Galik, B., Grimanelli, D., Grimwood, J., Grossniklaus, U., Hamada, T., Haseloff, J., Hetherington, A.J., Higo, A., Hirakawa, Y., Hundley, H.N., Ikeda, Y., Inoue, K., Inoue, S.-I., Ishida, S., Jia, Q.-D., Kakita, M., Kanazawa, T., Kawai, Y., Kawashima, T., Kennedy, M., Kinose, K., Kinoshita, T., Kohara, Y., Koide, E., Komatsu, K., Kopischke, S., Kubo, M., Kyozuka, J., Lagercrantz, U., Lin, S.-S., Lindquist, E., Lipzen, A.M., Lu, C.-W., De Luna, E., Martienssen, R.A., Minamino, N., Mizutani, M., Mizutani, M., Mochizuki, N., Monte, I., Mosher, R., Nagasaki, H., Nakagami, H., Naramoto, S., Nishitani, K., Ohtani, M., Okamoto, T., Okumura, M., Phillips, J., Pollak, B., Reinders, A., Rövekamp, M., Sano, R., Sawa, S., Schmid, M.W., Shirakawa, M., Solano, R., Spunde, A., Suetsugu, N., Sugano, S., Sugiyama, A., Sun, R., Suzuki, Y., Takenaka, M., Takezawa, D., Tomogane, H., Tsuzuki, M., Ueda, T., Umeda, M., Ward, J.M., Watanabe, Y., Yazaki, K., Yokoyama, R., Yoshitake, Y., Yotsui, I., Zachgo, S. & Schmutz, J. (2017) Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304. https://doi.org/10.1016/j.cell.2017.09.030
Carafa, A., Duckett, J.G. & Ligrone, R. (2003) Subterranean gametophytic axes in the primitive liverwort Haplomitrium harbour a unique type of endophytic association with aseptate fungi. New Phytologist 160: 185–197. https://doi.org/10.1046/j.1469-8137.2003.00849.x
Chambers, S.M., Williams, P.G., Seppelt, R.D. & Cairney, J.W.G. (1999) Molecular identification of Hymenoscyphus sp. from rhizoids of the leafy liverwort Cephaloziella exiliflora in Australia and Antarctica. Mycological Research 103: 286–288. https://doi.org/10.1017/S0953756298007217
Chang, Y., Desirò, A., Na, H., Sandor, L., Lipzen A., Clum, A., Barry, K., Grigoriev, I.V., Martin, F.M., Stajich, J.E., Smith, M.E., Bonito, G. & Spatafora, J.W. (2019) Phylogenomics of Endogonaceae and evolution of mycorrhizas within Mucoromycota. New Phytologist 222: 511–525. https://doi.org/10.1111/nph.15613
Chang, Y., Wang, S., Sekimoto, S., Aerts, A.L., Choi, C., Clum, A., LaButti, K.M., Lindquist, E.A., Ngan, C.Y., Ohm, R.A., Salamov, A.A., Grigoriev, I.V., Spatafora, J.W. & Berbee, M.L. (2015) Phylogenomic analyses indicate that early fungi evolved digesting cell walls of algal ancestors of land plants. Genome Biology and Evolution 7: 1590–1601. https://doi.org/10.1093/gbe/evv090
Costa, J.-L., Paulstrud, P., Rikkinen, J. & Lindblad, P. (2001) Genetic diversity of Nostoc symbionts endophytically associated with two bryophyte species. Applied and Environmental Microbiology 67: 4393–4396. https://doi.org/10.1128/aem.67.9.4393-4396.2001
Crandall-Stotler, B.R., Stotler, R.E. & Long, D.G. (2009a) Morphology and classification of the Marchantiophyta. In: Goffinet, B. & Shaw, A.J. (Eds.) Bryophyte Biology, 2nd edition. Cambridge University Press, Cambridge, pp. 1–54. https://doi.org/10.1017/CBO9780511754807.002
Crandall-Stotler, B., Stotler, R.E. & Long, D.G. (2009b) Phylogeny and classification of the Marchantiophyta. Edinburgh Journal of Botany 66: 155–198. https://doi.org/10.1017/S0960428609005393
Davis, E.C. (2004) Molecular phylogeny of leafy liverworts according to analysis of twelve genes. In: Hollowell, V. & Magill, R. (Eds.) Molecular Systematics of Bryophytes, Missouri Botanical Gardens Press, St. Louis, pp. 61–86.
Delaux, P.M., Radhakrishnan, G.V., Jayaraman, D., Cheema, J., Malbreil, M., Volkening, J.D., Sekimoto, H., Nishiyama, T., Melkonian, M., Pokorny, L. & Rothfels, C.J. (2015) Algal ancestor of land plants was preadapted for symbiosis. Proceedings of the National Academy of Sciences 112: 13390–13395. https://doi.org/10.1073/pnas.1515426112
Delaux, P.M., Séjalon-Delmas, N., Bécard, G. & Ané, J.M. (2013) Evolution of the plant–microbe symbiotic ‘toolkit’. Trends in Plant Science 18: 298–304. https://doi.org/10.1016/j.tplants.2013.01.008
De Roo, R.T., Hedderson, T.A. & Söderström, L. (2007) Molecular insights into the phylogeny of the leafy liverwort family Lophoziaceae Cavers. Taxon 56: 301–314. https://doi.org/10.1002/tax.562005
de Sousa, F., Foster, P.G., Donoghue, P.C.J., Schneider, H. & Cox, C.J. (2018) Nuclear protein phylogenies support the monophyly of the three bryophyte groups (Bryophyta Schimp.). New Phytologist 222: 565–575. https://doi.org/10.1111/nph.15587
Desirò, A., Duckett, J.G., Pressel, S., Villarreal, J.C. & Bidartondo, M.I. (2013) Fungal symbioses in hornworts: a chequered history. Proceedings of the Royal Society of London B. 280: 20130207. https://doi.org/10.1098/rspb.2013.0207
Duckett, J.G., Carafa, A. & Ligrone, R. (2006) A highly differentiated glomeromycotean association with the mucilage-secreting, primitive antipodean liverwort Treubia: clues to the origins of mycorrhizas. American Journal of Botany 93: 797–813. https://doi.org/10.3732/ajb.93.6.797
Duckett, J.G. & Ligrone, R. (1992) A light and electron microscope study of the fungal endophytes in the sporophyte and gametophyte of Lycopodium cernuum L. with observations on the gametophyte-sporophyte junction. Canadian Journal of Botany 70: 58–72. https://doi.org/10.1139/b92-008
Duckett, J.G. & Ligrone R. (2008a) A cytological analysis of basidiomycetous endophytes in New Zealand Aneuraceae (simple thalloid liverworts, Metzgeriidae); confirmation of the derived status of Verdoornia. Canadian Journal of Botany 86: 346–358. https://doi.org/10.1139/B08-004
Duckett, J.G. & Ligrone, R. (2008b) Endophytic fungi in New Zealand liverworts. In: Engel, J.J. & Glenny, D. (Eds.) A Flora of the Liverworts of New Zealand. Volume I. Missouri Botanical Garden Press, St. Louis. Monographs in Systematic Botany from the Missouri Botanical Garden 110, pp.48–56.
Duckett, J.G., Prasad, A.K.S.K., Davies, D.A. & Walker, S. (1977) A cytological analysis of the Nostoc-bryophyte relationship. New Phytologist 79: 349–362. https://doi.org/10.1111/j.1469-8137.1977.tb02215.x
Duckett, J.G. & Read, D.J. (1995) Ericoid mycorrhizas and rhizoid ascomycete associations in liverworts share the same mycobiont: isolation of the partners and resynthesis of the associations in vitro. New Phytologist 129: 439–447. https://doi.org/10.1111/j.1469-8137.1995.tb04315.x
Duckett, J.G., Renzaglia, K.S. & Pell, K. (1991) A light and electron microscope study of rhizoid ascomycete associations and flagelliform axes in British hepatics with observations on the effects of the fungi on host morphology. New Phytologist 118: 233–257. https://doi.org/10.1111/j.1469-8137.1991.tb00975.x
Duckett, J.G., Russell, J. & Ligrone, R. (2006b) Basidiomycetous endophytes in jungermannialean (leafy) liverworts have novel cytology and species-specific host ranges: a cytological and experimental study. Canadian Journal of Botany 84: 1075–1093. https://doi.org/10.1139/b06-073
Edwards, E., Cherns, L. & Raven, J.A. (2015) Could land-based early photosynthesizing ecosystems have bioengineered the planet in mid-Palaeozoic times? Palaeontology 58: 803–837. https://doi.org/10.1111/pala.12187
Engel, J.J. & Braggins, J.E. (2005) Are Mylia and Trabacellula (Hepaticae) related? Unsuspected links revealed by cell wall morphology, with the transfer of Mylia anomala to a new genus (Leiomylia J.J. Engels & Braggins) of Jungermanniaceae. Taxon 54: 665–680. https://doi.org/10.2307/25065423
Fehrer, J., Réblová, M., Bambasová, V. & Vohník, M. (2019) The root-symbiotic Rhizoscyphus ericae aggregate and Hyaloscypha (Leotiomycetes) are congeneric: Phylogenetic and experimental evidence. Studies in Mycology 92: 195–225. https://doi.org/10.1016/j.simyco.2018.10.004
Field, K.J., Cameron, D.D., Leake, J.R., Tille, S., Bidartondo, M.I. & Beerling, D.J. (2012) Contrasting arbuscular mycorrhizal responses of vascular and non-vascular plants to a simulated Palaeozoic CO2 decline. Nature Communications 3: 835. https://doi.org/10.1038/ncomms1831
Field, K.J., Bidartondo, M.I., Rimington, W.R., Hoysted, G., Beerling, D., Cameron, D., Duckett, J.G., Leake, J. & Pressel, S. (2019) Functional complementarity of ancient plant-fungal mutualisms: contrasting nitrogen, phosphorus and carbon exchanges between Mucoromycotina and Glomeromycotina fungal symbionts of liverworts. New Phytologist 223: 908–921. https://doi.org/10.1111/nph.15819
Field, K.J. & Pressel, S. (2018) Tansley Review - Unity in diversity: structural and functional insights into an ancient partnership between plants and fungi. New Phytologist 220: 996–1011. https://doi.org/10.1111/nph.15158
Field, K.J., Pressel, S., Duckett, J.G., Rimington, W.R. & Bidartondo, M.I. (2015b) Symbiotic options for the conquest of land. Trends in Ecology and Evolution 30: 477–486. https://doi.org/10.1016/j.tree.2015.05.007
Field, K.J., Rimington, W.R., Bidartondo, M.I., Allinson, K.E., Beerling, D.J., Cameron, D.D., Duckett, J.G., Leake, J.R. & Pressel, S. (2016) Functional analysis of liverworts in dual symbiosis with Glomeromycota and Mucoromycotina fungi under a simulated Palaeozoic CO2 decline. ISME Journal 10: 1514–1526. https://doi.org/10.1038/ismej.2015.204
Field, K.J., Rimington, W.R., Bidartondo, M.I., Allison, K.E., Beerling, D.J., Cameron, D.D., Duckett, J.G., Leake, J.R. & Pressel, S. (2015a) First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytologist 205: 743–756. https://doi.org/10.1111/nph.13024
Fisher, J.B. & Vovides, A.P. (2004) Mycorrhizae are present in cycad roots. The Botanical Review 70: 16–23. [https://www.jstor.org/stable/27571171]
Forrest, L.L. & Crandall-Stotler, B.J. (2004) A phylogeny of the simple thalloid liverworts (Jungermanniopsida, Metzgeriidae) as inferred from five chloroplast genes. In: Goffinet, B., Hollowell, V. & Magill, M.R. (Eds.) Molecular Systematics of Bryophytes. St. Louis, MO, USA: Missouri Botanical Garden, pp. 119–140.
Forrest, L.L., Davis, E.C., Long, D.G., Crandall-Stotler, B.J., Clark, A. & Hollingsworth, M.L. (2006) Unravelling the evolutionary history of the liverworts (Marchantiophyta): multiple taxa, genomes and analyses. Bryologist 109: 303–334. https://doi.org/10.1639/0007-2745(2006)109[303:UTEHOT]2.0.CO;2
Frangedakis, E., Shimamura, M., Villarreal, J.C., Li, F-W.& Tomaselli, M., Waller, M., Kakakibara, K., Renzaglia, K.S. & Szövényi, P. (2021) The hornworts: morphology, evolution and development. New Phytologist 229: 735–754. https://doi.org/10.1111/nph.16874
Gottsche, C.M. (1843) Anatomisch-physiologische Untersuchungen über Haplomitrium hookeri N.v.E mit Vergleichung anderer Lebermoose. Novorum actorum Academia Caesareae Leopoldinae-Carolinae Germanicae Naturae Curiosorum 20: 267–398.
Grolle, R. (1963) Zwei Gattungen der Lophoziaceae neu für Afrika. Transactions of the British Bryological Society 4: 437–445. https://doi.org/10.1179/006813863804812363
Hambleton, S. & Sigler, L. (2005) Meliniomyces, a new anamorph genus for root-associated fungi with phylogenetic affinities to Rhizoscyphus ericae (=Hymenoscyphus ericae), Leotiomycetes. Studies in Mycology 53: 1–27. https://doi.org/10.3114/sim.53.1.1
Harris, B.J., Harrison, C.J., Hetherington, A.M. & Williams, T.A. (2020) Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Current Biology 30: 2001–2012.e2. https://doi.org/10.1016/j.cub.2020.03.048
Hentschel, J., Wilson, R., Burghardt, M., Zündorf, H.-J., Schneider, H. & Heinrichs, J. (2006) Reinstatement of Lophocoleaceae (Jungermanniopsida) based on chloroplast gene rbcL data: exploring the importance of female involucres for the systematics of Jungermanniales. Plant Systematics and Evolution 258: 211–226. https://doi.org/10.1007/s00606-006-0408-y
Hentschel, J., Feldberg, K., Zündorf, H.-J., Hellwig, F.H., Schneider, H. & Heinrichs, J. (2007) The systematic position of Pachyglossa and Clasmatocolea (Jungermanniopsida: Lophocoleaceae) inferred from nrDNA ITS sequences and morphology. Taxon 56: 1136–1142. https://doi.org/10.2307/25065908
He-Nygren, X., Ahonen, I., Juslen, A., Glenny, D. & Piippo, S. (2004) Phylogeny of liverworts – beyond a leaf and a thallus. In: Goffinet, B., Hollowell, V. & Magill, M.R. (Eds.) Molecular Systematics of Bryophytes. Missouri Botanical Garden, St. Louis, MO, USA, pp. 87–118.
Hoysted, G.A., Bidartondo, M.I, Duckett, J.G., Pressel, S. & Field, K.J. (2021) Phenology and function in lycopod-Mucoromycotina symbiosis. New Phytologist – Letter 229: 2389–2394. https://doi.org/10.1111/nph.17009
Hoysted, G.A., Jacob, A.S., Kowal, J., Giesemann, P., Bidartondo, M.I., Duckett, J.G., Gebauer, G., Rimington, W.R., Schornack, S., Pressel, S. & Field, K.J. (2019) Mucoromycotina fine root endophyte fungi form nutritional mutualisms with vascular plants. Plant Physiology 181: 565–577. https://doi.org/10.1104/pp.19.00729
Humphreys, C.P., Franks, P.J., Rees, M., Bidartondo, M.I., Leake, J.R. & Beerling, D.J. (2010) Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nature Communications 1: 103. https://doi.org/10.1038/ncomms1105
Kottke, I., Beiter, A., Weiss, M., Haug, I., Oberwinkler, F. & Nebel, M. (2003) Heterobasidiomycetes from symbiotic associations with hepatics: Jungermanniales have sebacinoid mycobionts while Aneura pinguis (Metzgeriales) is associated with a Tulasnella species. Mycological Research 107: 957–968. https://doi.org/10.1017/s0953756203008141
Kottke, I. & Nebel, M. (2005) The evolution of mycorrhiza-like associations in liverworts: an update. New Phytologist 167: 330–334. https://doi.org/10.1111/j.1469–8137.2005.01471.x
Kowal, J., Arrigoni, E., Jordi, S. & Bidartondo, M.I. (2020) Prevalence and phenology of fine endophyte colonisation across populations of Lycopodiella inundata. Mycorrhiza 30: 577–587. http://dx.doi.org/10.1007/s00572-020-00979-3
Kowal, J., Pressel, S., Duckett, J.G., Bidartondo, M.I., Field, K.J. (2018) From rhizoids to roots? Experimental evidence of mutualism between liverworts and ascomycete fungi. Annals of Botany 121: 221–227. https://doi.org/10.1093/aob/mcx126
Koske, R.E., Gemma, J.N. & Doyle, M.F. (1992) Mycorrhizal status of Gunnera petaloidea in Hawaii. Pacific Science 46: 480–483.
Krause, C., Sigisfredo, G., Bauer, R. & Nebel, M. (2011) Aneuraceae (Metzgeriales) and tulasnelloid fungi (Basidiomycota) – a model for early steps in fungal symbiosis. Fungal Biology 115: 839–851. https://doi.org/10.1016/j.funbio.2011.06.013
Krings, M., Hass, H., Kerp, H., Taylor, T.N., Agerer, R. & Dotzler, N. (2009) Endophytic cyanobacteria in a 400-million-yr-old land plant: A scenario for the origin of a symbiosis? Review of Paleobotany and Palynology 153: 62–69. https://doi.org/10.1016/j.revpalbo.2008.06.006
Krings, M., Taylor, T.N. & Dotzler, N. (2012) Fungal endophytes as a driving force in land plant evolution. In: Southworth, D. (Ed.) Biocomplexity of Plant-Fungal Interactions. John Wiley & Sons, pp. 5–28. https://doi.org/10.1002/9781118314364.ch1
Krings, M., Taylor, T.N., Hass, H., Kerp, H., Dotzler, N. & Hermsen, E.J. (2007) Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytologist 175: 648–657. https://doi.org/10.1111/j.1469-8137.2007.02008.x
James, T.Y., Kauff, F., Schoch, C.L., Matheny, P.B., Hofstetter, V., Cox, C.J., Celio, G., Gueidan, C., Fraker, E., Miadlikowska, J., Lumbsch, H.T., Rauhut, A., Reeb, V., Arnold, A.E., Amtoft, A., Stajich, J.E., Hosaka, K., Sung, G.-H., Johnson, D., O’Rourke, B., Crockett, M., Binder, M., Curtis, J.M., Slot, J.C., Wang, Z., Wilson, A.W., Schüßler, A., Longcore, J.E., O’Donnell, K., Mozley-Standridge, S., Porter, D., Letcher, P.M., Powell, M.J., Taylor, J.W., White, M.M., Griffith, G.W., Davies, D.R., Humber, R.A., Morton, J.B., Sugiyama, J., Rossman, A.Y., Rogers, J.D., Pfister, D.H., Hewitt, D., Hansen, K., Hambleton, S., Shoemaker, R.A., Kohlmeyer, J., Volkmann-Kohlmeyer, B., Spotts, R.A., Serdani, M., Crous, P.W., Hughes, K.W., Matsuura, K., Langer, E., Langer, G., Untereiner, W.A., Lücking, R., Büdel, B., Geiser, D.M., Aptroot, A., Diederich, P., Schmitt, I., Schultz, M., Yahr, R., Hibbett, D.S., Lutzoni, F., McLaughlin, D.J., Spatafora, J.W. & Vilgalys, R. (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443: 818–822. https://doi.org/10.1038/nature05110
Jia, Y., Gray, V.M. & Straker, C.J. (2004) The influence of Rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Vicia faba. Annals of Botany 94: 251–258. https://doi.org/10.1093/aob/mch135
Lee, S-J., Kong, M., Harrison, P. & Hijri, M. (2018) Conserved proteins of the RNA interference system in the arbuscular mycorrhizal fungus Rhizoglomus irregulare provide new insight into the evolutionary history of Glomeromycota. Genome Biology and Evolution 10: 328–343. https://doi.org/10.1093/gbe/evy002
Lenton, T.M. & Daines, S.J. (2017) Matworld – the biogeochemical effects of early life on land. New Phytologist 215: 531–537. https://doi.org/10.1111/nph.14338
Li, F-W., Nishiyama, T., Waller, M., Frangedakis, E., Keller, J., Li, Z., Fernandez-Pozo, N., Barker, M.S., Bennett, T., Blázquez, M.A., Cheng, S., Cuming, A.C., de Vries, J., de Vries, S., Delaux, P.-M., Diop, I.S., Harrison, C.J., Hauser, D., Hernández-García, J., Kirbis, A., Meeks, J.C., Monte, I., Mutte, S.K., Neubauer, A., Quandt, D., Robison, T., Shimamura, M., Rensing, S.A., Villarreal, J.C., Weijers, D., Wicke, S., Wong, G.K.-S., Sakakibara, K. & Szövényi, P. (2020) Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nature Plants 6: 259–272. https://doi.org/10.1038/s41477-020-0618-2
Ligrone, R. (1988) Ultrastructural of a fungal endophyte in Phaeoceros laevis (L.) Prosk. (Anthocerotophyta). Botanical Gazette 149: 92–100. [https://www.jstor.org/stable/2995425]
Ligrone, R., Carafa, A., Lumini, E., Bonfante, P., Biancotto, V. & Duckett, J.G. (2007) Glomeromycotean associations in liverworts: a molecular, cytological and taxonomical survey. American Journal of Botany 94: 1756–1777. https://doi.org/10.3732/ajb.94.11.1756
Lindblad, P. & Bergman, B. (2018) The cycad-cyanobacterial symbiosis. In: Rai, A.N. (Ed.) CRC handbook of symbiotic cyanobacteria. CRC Press, Boca Raton, FL, USA, 137–159.
Margulis, L. & Chapman, M.J. (1998) Endosymbioses: cyclical and permanent evolution. Trends in Microbiology 6: 342–345. https://doi.org/10.1016/S0966-842X(98)01325-0
Nebel, M., Kreier, H.-P., Preußing, M. & Weiss, M.A. (2004) Symbiotic fungal associations with liverworts are the possible ancestors of mycorrhizae. In: Agerer, R., Piepenbring, H. & Blanz, P. (Eds.) Frontiers in Basidiomycote Mycology, HIW-Verlag, Ecking, Germany, pp. 339–360.
Newsham, K.K. & Bridge, P.D. (2010) Sebacinales are associates of the leafy liverwort Lophozia excisa in the southern maritime Antarctic. Mycorrhiza 20: 307–313. https://doi.org/10.1007/s00572-009-0283-9
Oldroyd, G.E. (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nature Reviews Microbiology 11: 252–263. https://doi.org/10.1038/nrmicro2990
Orchard, S., Hilton, S., Bending, G.D., Dickie, I.A., Standish, R.J., Gleeson, D.B., Jeffery, R.P., Powell, J.R., Walker, C., Bass, D., Monk, J., Simonin, A. & Ryan, M.H. (2017a) Fine endophytes (Glomus tenue) are related to Mucoromycotina, not Glomeromycotina. New Phytologist 213: 481–486. https://doi.org/10.1111/nph.14268
Orchard, S., Standish, R.J., Dickie, I.A., Renton, M., Walker, C., Moot, D. & Ryan, M.H. (2017b) Fine root endophytes under scrutiny: a review of the literature on arbuscule-producing fungi recently suggested to belong to the Mucoromycotina. Mycorrhiza 27: 619–638. https://doi.org/10.1007/s00572-017-0782-z
Paton, J.A. (1999) The Liverwort Flora of the British Isles. Brill, Leiden.
Perotto, S., Daghino, S. & Martino, E. (2018) Ericoid mycorrhizal fungi and their genomes: another side to mycorrhizal symbiosis? New Phytologist 220: 1141–1147. https://doi.org/10.1111/nph.15218
Pirozynski, K.A. & Malloch, D.W. (1975) The origin of land plants: a matter of mycotrophism. Biosystems 6: 153–164. https://doi.org/10.1016/0303-2647(75)90023-4
Pressel, S., Bidartondo, M.I., Field, K.J., Rimington, W.R. & Duckett, J.G. (2016) Pteridophyte fungal associations: Current knowledge and future perspectives. Journal of Systematics and Evolution 54: 666–678. https://doi.org/10.1111/jse.12227
Pressel, S., Bidartondo, M.I., Ligrone, R. & Duckett, J.G. (2010) Fungal symbioses in bryophytes: New insights in the twenty first century. Phytotaxa 9: 238–253. https://doi.org/10.11646/phytotaxa.9.1.13
Pressel, S., Davis, E.C., Ligrone, R. & Duckett, J.G. (2008a) An ascomycetous endophyte induces branching and septation of the rhizoids in the leafy liverwort family the Schistochilaceae (Jungermanniidae, Hepaticopsida). American Journal of Botany 95: 531–541. https://doi.org/10.3732/ajb.2007171
Pressel, S., Ligrone, R. & Duckett, J.G. (2008b) The ascomycete Rhizoscyphus ericae elicits a range of host responses in the rhizoids of leafy liverworts; an experimental and cytological analysis. Fieldiana 47: 59–72. https://doi.org/10.3158/0015-0746-47.1.59
Pressel, S., P’ng, K.M.Y. & Duckett, J.G. (2011) An ultrastructural study of the liverwort Mizutania riccardioides Furuki et Iwaksuki: new insights into its systematic affinities and unique surface ornamentation. Bryologist 114: 38–51.
Preußing, M., Nebel, M., Oberwinkler, M. & Weiss, M. (2010) Diverging diversity patterns in Tulasnella (Basidiomycota, Tulasnellales) mycobionts of Aneura pinguis (Marchantiophyta, Metzgeriales) from Europe and Ecuador. Mycorrhiza 20: 147–159. https://doi.org/10.1007/s00572-009-0275-9
Puttick, M.N., Morris, J.L., Williams, T.A., Cox, C.J., Edwards, D., Kenrick, P., Pressel, S., Wellman, C.H., Schneider, H., Pisani, D. & Donoghue, P.C.J. (2018) The interrelationships of land plants and the nature of the ancestral embryophyte. Current Biology 28: 733–745.e2. https://doi.org/10.1016/j.cub.2018.01.063
Qiu, Y-L., Li, L., Wang, B., Chen, Z., Knoop, V., Groth-Malonek, M., Dombrovska, O., Lee, J., Kent, L., Rest, J., Estabrook, J.F., Hendry, T.A., Taylor, D.W., Testa, C.M., Ambros, M., Crandall-Stotler, B., Duff, R.J., Stech, M., Frey, W., Quandt, D. & Davis, C.C. (2006) The deepest divergences in land plants inferred from phylogenomic evidence. Proceedings of the National Academy of Sciences, USA 103: 15511–15516. https://doi.org/10.1073/pnas.0603335103
Read, D.J., Duckett, J.G., Francis, R., Ligrone, R. & Russell, A. (2000) Symbiotic fungal associations in ‘lower’ land plants. Philosophical Transactions of the Royal Society B. 355: 815–832. [https://www.jstor.org/stable/3066807]
Read, D.J., Leake, J.R. & Perez-Moreno, J. (2004) Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Canadian Journal of Botany 82: 1243–1263. https://doi.org/10.1139/b04-123
Remy, W., Taylor, T.N., Hass, H. & Kerp, H. (1994) Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences, USA 91: 11841–11843. https://doi.org/10.1073/pnas.91.25.11841
Renzaglia, K.S., Villarreal, J.C. & Duff, RJ. (2009) New insights into morphology, anatomy, and systematics of hornworts. In: Goffinet, B. & Shaw, A.J. (Eds.) Bryophyte Biology. Cambridge University Press, Cambridge, pp. 139–171. https://doi.org/10.1017/CBO9780511754807.004
Rikkinen, J. (2017) Cyanobacteria in terrestrial symbiotic systems. In: Hallenbeck, P. (ed.) Modern Topics in the Phototrophic Prokaryotes, Springer, Cham, pp. 243–294. https://doi.org/ 10.1007/978-3-319-46261-5_8
Rimington, W.R., Duckett, J.G., Field, K.J., Bidartondo, M.I. & Pressel, S. (2020) The distribution and evolution of fungal symbioses in ancient lineages of land plants. Mycorrhiza 30: 23–49. https://doi.org/10.1007/s00572-020-00938-y
Rimington, W.R., Pressel, S., Duckett, J.G., Field, K.J. & Bidartondo, M.I. (2019) Evolution and networks in ancient and widespread symbioses between Mucoromycotina and liverworts. Mycorrhiza 29: 551–565. https://doi.org/10.1007/s00572-019-00918-x
Rimington, W.R., Pressel, S., Duckett, J.G., Field, K.J., Read, D.J. & Bidartondo, M.I. (2018) Ancient plants with ancient fungi: liverworts associate with early-diverging arbuscular mycorrhizal fungi. Proceedings of the Royal Society B: Biological Sciences 285: 20181600. https://doi.org/10.1098/rspb.2018.1600
Russell, J. & Bulman, S. (2005) The liverwort Marchantia foliacea forms a specialized symbiosis with arbuscular mycorrhizal fungi in the genus Glomus. New Phytologist 165: 567–579. https://doi.org/10.1111/j.1469-8137.2004.01251.x
Schmid, D. & Oberwinkler, F. (1993) Mycorrhiza-like interaction between the achlorophyllous gametophyte of Lycopodium clavatum L. and its fungal endophyte studied by light and electron microscopy. New Phytologist 124: 69–81. https://doi.org/10.1111/j.1469-8137.1993.tb03798.x
Schüßler, A. (2000) Glomus claroideum forms an arbuscular mycorrhiza-like symbiosis with the hornwort Anthoceros punctatus. Mycorrhiza 10: 15–21. https://doi.org/10.1007/s005720050282
Schüßler, A. (2002) Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant and Soil 244: 75–83. https://doi.org/10.1023/A:1020238728910
Schüßler, A. (2012) 5. The Geosiphon–Nostoc endosymbiosis and its role as a model for arbuscular mycorrhiza research. In: Hock, B. (Ed.) Fungal Associations. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research), vol. 9. Springer, Berlin, Heidelberg, pp. 77–91. https://doi.org/10.1007/978-3-642-30826-0_5
Schüßler, A., Schwarzott, D. & Walker, C. (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research 105: 1413–1421. https://doi.org/10.1017/S0953756201005196
Schüßler, A. & Walker, C. (2011) Evolution of the ‘plant-symbiotic’ fungal phylum, Glomeromycota. In: Pöggeler, S. & Wöstemeyer (Eds.) The Mycota XIV. Evolution of Fungi and Fungal-Like Organisms. Springer, Berlin, pp. 163–185. https://doi.org/10.1007/978-3-642-19974-5_7
Schuster, R.M. (1966) The Hepaticae and Anthocerotae of North America. Volume 1. Colombia University Press, New York.
Schuster, R.M. (1969) The Hepaticae and Anthocerotae of North America. II. Columbia University Press, New York.
Shaw, B., Crandall-Stotler, B., Váňa, J., Stotler, R.E., von Konrat, M., Engel, J.J., Davis, E.C., Long, D.G., Sova, P. & Shaw, A.J. (2015) Phylogenetic relationships and morphological evolution in a major clade of leafy liverworts (phylum Marchantiophyta, order Jungermanniales): suborder Jungermanniineae. Systematic Botany 40: 27–45. https://doi.org/10.1600/036364415X68314
Selosse, M.-A. (2005) Are liverworts imitating mycorrhizas? New Phytologist 165: 345–349.
Smith, F.A. & Smith, S.E. (1997) Structural diversity in (vesicular)–arbuscular mycorrhizal symbioses. New Phytologist 137: 373–388. https://doi.org/10.1046/j.1469-8137.1997.00848.x
Spatafora, J.W., Chang, Y., Benny, G.L., Lazarus, K., Smith, M.E., Berbee, M.L., Bonito, G., Corradi, N., Grigoriev, I., Gryganskyi, A. & James, T.Y. (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108: 1028–1046. https://doi.org/10.3852/16-042
Sousa, F., Civáň, P., Brazão, J., Foster, P.G. & Cox, C.J. (2020) The mitochondrial phylogeny of land plants shows support for Setaphyta under composition-heterogeneous substitution models. PeerJ 8: e8995. http://doi.org/10.717/peerj.8995
Söderstrom, L., Hagborg, A., von Konrat, M., Bartholomew-Began, S., Bell, D., Briscoe, L., Brown, E., Cargill, D.C., Costa, D.P., Crandall-Stotler, B.J., Cooper, E.D., Dauphin, G., Engel, J., Feldberg, K., Glenny, D., Gradstein, S.R., He, X.-L., Heinrichs, J., Hentschel, J., Ilkiu-Borges, A.L., Katagiri, T., Konstantinova, N.A., Larraín, J., Long, D., Nebel, M., Pócs, T., Puche, F., Reiner-Drehwald, E., Renner, M., Sass-Gyarmati, A., Schäfer-Verwimp, A., Segarra-Moragues, J.G., Stotler, R.E., Sukkharak, P., Thiers, B., Uribe, J., Váňa, J., Villarreal, J., Wigginton, M., Zhang, L. & Zhu, R.-L. (2016) World checklist of hornworts and liverworts. Phytokeys 59: 1–828. https://doi.org/10.3897/phytokeys.59.6261
Stahl, M. (1949) Die Mycorrhiza der Lebermoose mit besonderer Berücksichtigung der thallosen Formen. Planta 37: 103–148. [https://www.jstor.org/stable/23359038]
Strullu-Derrien, C., Kenrick, P., Pressel, S., Duckett, J.G., Rioult, J.P. & Strullu, D.G. (2014) Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million ear old) closely resemble those in extant lower land plants: novel insights into ancestral plant–fungus symbioses. New Phytologist 203: 964–979. https://doi.org/10.1111/nph.12805
Szövényi, P. (2016) The genome of the model species Anthoceros agrestis. In: Rensing, SA. (Ed.) Genomes and Evolution of Charophytes, Bryophytes, Lycophytes and Ferns. Elsevier, Amsterdam, pp.189–211. https://doi.org/10.1016/bs.abr.2015.12.001
Szövényi, P., Frangedakis, E., Ricca, M., Quandt, D., Wicke, S. & Langdale, J.A. (2015) Establishment of Anthoceros agrestis as a model species for studying the biology of hornworts. BMC Plant Biology 15: 98. https://doi.org/10.1186/s12870-015-0481-x
Taylor, T.N., Remy, W., Hass, H. & Kerp, H. (1995) Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87: 560–573. https://doi.org/10.2307/3760776
Tedersoo, L., Pärtel, K., Jairus, T., Gates, G., Poldmaa, K. & Tamm, H. (2009) Ascomycetes associated with ectomycorrhizas: molecular diversity and ecology with particular reference to the Helotiales. Environmental Microbiology 11: 3166–3178. https://doi.org/10.1111/j.1462-2920.2009.02020.x
Uehling, J., Gryganskyi, A., Hameed, K., Tschaplinski, T., Misztal, P.K., Wu, S., Desirò, A., Vande Pol, N., Du, Z., Zienkiewicz, A. & Zienkiewicz, K. (2017) Comparative genomics of Mortierella elongata and its bacterial endosymbiont Mycoavidus cysteinexigens. Environmental Microbiology 19: 2964–2983. https://doi.org/10.1111/1462-2920.13669
Upson, R., Read, D.J. & Newsham, K.K. (2007) Widespread association between the ericoid mycorrhizal fungus Rhizoscyphus ericae and a leafy liverwort in the maritime and sub-Antarctic. New Phytologist 176: 460–471. https://doi.org/10.1111/j.1469-8137.2007.02178.x
Villarreal, J.C. & Renner, S.S. (2012) Hornwort pyrenoids, carbon-concentrating structures, evolved and were lost at least five times during the last 100 million years. Proceedings of the National Academy of Sciences, USA 109: 18873–18878. https://doi.org/10.1073/pnas.1213498109
Villarreal, J.C. & Renzaglia, KS. (2006) Structure and development of Nostoc strands in Leiosporoceros dussii (Anthocerotophyta): a novel symbiosis in land plants. American Journal of Botany 93: 693–705. https://doi.org/10.3732/ajb.93.5.693
Villarreal-Ruiz, L., Anderson, I.C. & Alexander, I.J. (2004) Interactions between an isolate from the Hymenoscyphus ericae aggregate and roots of Pinus and Vaccinium. New Phytologist 164: 183–192. https://doi.org/10.1111/j.1469-8137.2004.01167.x
Walker, C., Gollotte, A. & Redecker, D. (2018) A new genus, Planticonsortium (Mucoromycotina), and new combination (P. tenue), for the fine root endophyte, Glomus tenue (basionym Rhizophagus tenuis). Mycorrhiza 28: 213–219. https://doi.org/10.1007/s00572-017-0815-7
Wang, B. & Qiu, Y.L. (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16: 299–363. https://doi.org/10.1007/s00572-005-0033-6
Wang, B., Yeun, L.H., Xue, J.Y., Liu, Y., Ané, J.M. & Qiu, Y.L. (2010) Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytologist 186: 514–525. https://doi.org/10.1111/j.1469-8137.2009.03137.x
Warshan, D., Liaimer, A., Pederson, E., Kim, S-Y., Shapiro, N., Woyke, T., Altermark, B., Pawloski, K., Weyman, P.D., Dupont, C.L. & Rasmussen, U. (2018) Genomic changes associated with the evolutionary transitions of Nostoc to a plant symbiont. Molecular Biology and Evolution 35: 1160–1175. https://doi.org/10.1093/molbev/msy029
Wickett, N.J., Mirarab, S., Nguyen, N., Warnow, T., Carpenter, E., Matasci, N., Ayyampalayam, S., Barker, M.S., Burleigh, J.G., Gitzendanner, M.A., Ruhfel, B.R., Wafula, E., Der, J.P., Graham, S.W., Mathews, S., Melkonian, M., Soltis, D.E., Soltis, P.S., Miles, N.W., Rothfels, C.J., Pokorny, L., Shaw, A.J., DeGironimo, L., Stevenson, D.W., Surek, B., Villarreal, J.C., Roure, B., Philippe, H., dePamphilis, C.W., Chen, T., Deyholos, M.K., Baucom, R.S., Kutchan, T.M., Augustin, M.M., Wang, J., Zhang, Y., Tian, Z., Yan, Z., Wu, X., Sun, X., Wong, G.K.-S. & Leebens-Mack, J. (2014) Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences, USA 111: E4859–E4868. https://doi.org/10.1073/pnas.1323926111
Whitton, B.A. (1993) Cyanobacteria and Azolla. In: Jones, D.G. (Ed.) Exploitation of microorganisms. Springer, Dordrecht, pp. 137–167. https://doi.org/10.1007/978-94-011-1532-2_6
Xavier, L.J.C. & Germida, J.J. (2002) Response of lentil under controlled conditions to co-inoculation with arbuscular mycorrhizal fungi and rhizobia varying in efficacy. Soil Biology & Biochemistry 34: 181–188. https://doi.org/10.1016/S0038-0717(01)00165-1
Xavier, L.J.C. & Germida, J.J. (2003) Selective interactions between arbuscular mycorrhizal fungi and Rhizobium leguminosarum bv. viceae enhance pea yield and nutrition. Biology and Fertility of Soils 37: 262–267. https://doi.org/10.1007/s00374-003-0605-6