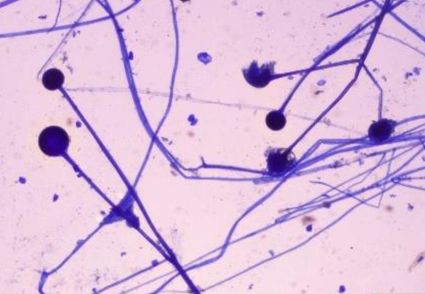
Abstract
Sri Lanka as an agricultural country requires seed health testing to manage crop diseases. So far no comprehensive research has been carried out for the proper identification of seed mycoflora affecting the seed quality in Sri Lanka. The present study strives to address this issue by identifying seed-borne fungal pathogens from stored seeds using morpho-molecular characterization. Fungal pathogens were isolated from surface-sterilized and non-surface sterilized seeds of Arachis hypogea, Oryza sativa, Vigna radiata, and Vigna sinensis. PCR amplification and DNA sequencing of the internal transcribed spacer (ITS) region was carried out for molecular identification of pathogens. The germination quality of each seed variety was calculated by pot experiments. Distribution frequencies, percentage of germination, and seedling vigor were calculated and analyzed for each seed variety tested. In total eighteen isolates were recovered from the four seed varieties. Molecular characterization revealed that the fungal isolates recovered from all the four seed varieties belong to seven genera: Aspergillus, Bipolaris, Daldinia, Macrophomina, Orbilia, Rhizopus, and Talaromyces. Rhizopus spp. showed the highest distribution frequency (75%). Arachis hypogea showed the lowest germination percentage (20%) and lowest seedling vigor index (585). The seeds of Oryza sativa showed no germination probably due to their high incidence of fungal pathogens (four out of seven genera). This study is the first comprehensive study analyzing the seed-borne pathogens of the four most commonly consumed Sri Lankan cereal and legume crops. Results reported in this study helps to identify and implement optimum storage facilities and control such fungal pathogens in future agricultural practices.
References
<p>Astoreca, A.L, Emateguy, L.G. & Alconada, T.M. (2019) Fungal contamination and mycotoxins associated with sorghum crop: its relevance today. <em>European Journal of Plant Pathology </em>155: 381–392. https://doi.org/10.1007/s10658-019-01797-w</p>
<p>Baka, Z.A.M., Serag, M.S. & Kardosha, T.A. (2014) Mycoflora associated with some stored seeds and their control by aqueous extract from medicinal plants. <em>Life Sciences Leaflets</em> 57: 49–62.</p>
<p>Baynes, M., Newcombe, G., Dixon, L., Castlebury, L. & O’Donnell, K. (2012) A novel plant-fungal mutualism associated with fire. <em>Fungal Biology</em> 116: 133–144. https://doi.org/10.1016/j.funbio.2011.10.008</p>
<p>Bhalerao, V.A. & Chavan., A.M. (2017) Isolation of Filamentous Fungi in Post-Harvest Cereal Grain During the Storage and Their Effect on Seed Health. <em>International Journal of Advanced Research</em> 5: 2171–2177. https://doi.org/10.21474/IJAR01/5283</p>
<p>Biswal, B.K., Jadhav, U.U., Madhaiyan, M., Ji, L., Yang, E. & Cao, B. (2018) Biological Leaching and Chemical Precipitation methods for recovery of Co and Li from spent Lithium-ion batteries. <em>Sustainable Chemistry and Engineering</em> 6: 12343–12352. https://doi.org/10.1021/acssuschemeng.8b02810</p>
<p>Chaudhari, A., Sharma, H., Jehani, M. & Sharma, J.K (2017) Seed mycoflora associated with pigeonpea [<em>Cajanus cajan</em> (L.) Millsp.], their Significance and the Management. <em>Journal of Pure and Applied Microbiology</em> 11: 567–575. https://doi.org/10.22207/JPAM.11.1.74</p>
<p>Chethana, K.W., Manawasinghe, I.S., Hurdeal, V.G., Bhunjun, C.S., Appadoo, M.A., Gentekaki, E., Raspe, O., Promputtha, I. & Hyde, K.D. (2021) What are fungal species and how to delineate them? <em>Fungal Diversity </em>109: 1–25. https://doi.org/10.1007/s13225-021-00483-9</p>
<p>Chowdhary, A., Kathuria, S., Singh, P.K., Sharma, B., Dolatabadi, S., Hagen, F. & Meis, J.F. (2014) Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. <em>Mycoses</em> 57: 97–107. https://doi.org/10.1111/myc.12234</p>
<p>Chutulo, E.C. & Chalannavar, R.K. (2020) <em>Daldinia eschscholtzii</em>: an endophytic fungus isolated from Psidium guajava as an alternative source of bioactive secondary metabolites. Asian Journal of Mycology 3: 376–398. https://doi.org/10.5943/ajom/3/1/11</p>
<p>Daranagama, D.A., Camporesi, E., Tian, Q., Li, X., Chamyuang, S., Stadler, M. & Hyde, K.D. (2015) <em>Anthostomella</em> is polyphyletic comprising several genera in <em>Xylariaceae.</em> <em>Fungal Diversity</em> 73: 203–238. https://doi.org/10.1007/s13225-015-0329-6</p>
<p>Dauda, W.P., Alao, S.E.L., Zarafi, A.B. & Alabi, O. (2017) Detection and Identification of seed-borne fungi on farmer saved seeds of onions (<em>Allium cepa</em>) in Kebbi state, Northwest Nigeria. <em>Journal of Agricultural Science</em> 3: 18–24.</p>
<p>de Carvalho, H.P., Mesquita, N., Trovao, J., da Silva, J.P., Rosa, B., Martins, R., Bandeira, A.M.L. & Portugal, A. (2016) Diversity of fungal species in ancient parchments collections of the archive of the University of Coimbra. <em>International Biodeterioration and Biodegradation</em> 108: 57–66. https://doi.org/10.1016/j.ibiod.2015.12.001</p>
<p>Deshabandu, K.H.S.T., Dissanayake, C.Y., Ekanayake, E.M.S.P., Weerakoon, W.M.W., Nijamudeen, M.S., Egodawatta, W.C.P. & Kumararathna, P<em>.</em> (2019) Screening of mungbean (<em>Vigna radiata</em>) accessions under intermittent drought stress. <em>Proceedings of the 2nd International Symposium on Agriculture</em> 174–185.</p>
<p>Di Piazza, S., Houbraken, J., Meijer, M., Cecchhi, G., Kraak, B., Rosa, E. & Zotti, M. (2020) Thermotolerant and Thermophilic Mycobiota in different steps of compost maturation. <em>Microorganisms</em> 880: 1–9. https://doi.org/10.3390/microorganisms8060880</p>
<p>Farley, G.J., Bellairs, S.M. & Adkins, S.W. (2013) Germination of selected Australian native grass species, with potential for minesite rehabilitation. <em>Australian Journal of Botany</em> 61: 283–290. https://doi.org/10.1071/BT12258</p>
<p>Fleurat-Lessard, F. (2017) Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins – An update. <em>Journal of Stored Products Research</em> 71: 22–40. https://doi.org/10.1016/j.jspr.2016.10.002</p>
<p>George, T., Dania, V.O. & Ikotun,T. (2019) Evaluation of seed-borne fungi associated with paddy rice (<em>Oryza sativa</em> L.) varieties using different isolation methods. <em>Nigerian Journal of Mycology </em>11: 46–58.</p>
<p>Ghosh, T., Biswas, M.K., Guin, C., Roy, P. & Aikat, K. (2018) A review on seed borne mycoflora associated with different cereal crop seeds and their management. <em>Plant Cell Biotechnology and Molecular Biology</em> 19: 107–117.</p>
<p>Gnanesh, B.N., Tejaswi, A., Arunakumar, G.S., Supriya, M., Manojkumar, H.B. & Tewary, P. (2021) Molecular phylogeny, identification and pathogenicity of <em>Rhizopus oryzae</em> associated with root rot of mulberry in India. <em>Journal of Applied Microbiology</em> 131: 360–374. https://doi.org/10.1111/jam.14959</p>
<p>Hughey, J.R. & Miller, K.A. (2016) Molecular phylogenetic analysis of <em>Sciadophycus stellatus</em> (Rhodymeniales, Rhodophyta) supports its placement in the family Rhodymeniaceae. <em>Phytotaxa</em> 245: 297–300. https://doi.org/10.11646/phytotaxa.245.4.7</p>
<p>Kritzinger, Q., Aveling, T.A.S., Marasas, W.F.O., Rheeder, J.P., Westhuizen, L.V.D. & Shepherd, G.S.<em>.</em> (2003) Mycoflora and fumonisin mycotoxins associated with cowpea [<em>Vigna unguiculat</em>a (L.) Walp] seeds. <em>Journal of Agricultural and Food Chemistry</em> 51: 2188–2192. https://doi.org/10.1021/jf026121v</p>
<p>Kumar, S., Lal, G.M. & Rai, P.K. (2017) Effects of seed treatments with botanical, chemical, on seed yield and quality traits in groundnut (<em>Arachis hypogea</em> L.). <em>Journal of Pharmacognosy and Phytochemistry</em> 6: 10–13.</p>
<p>Levic, J., Stankovic, S., Krnjaja, V., Bocarov-Stancic, A. & Ivanovic, D. (2012) Distribution frequency and incidence of seed-borne pathogens of some cereals and industrial crops in Serbia. <em>Pesticides and Phytomedicine</em> 27: 33–40. https://doi.org/10.2298/PIF1201033L</p>
<p>Maduranga, K., Attanayake, R.N., Santhirasegaram, S., Weerakoon, G. & Paranagama., P.A. (2018) Molecular phylogeny and bioprospecting of Endolichenic Fungi (ELF) inhabiting in the lichens collected from a mangrove ecosystem in Sri Lanka. <em>PLoS ONE</em> 13: 1–22. https://doi.org/10.1371/journal.pone.0200711</p>
<p>Millawithanachchi, M.C., Sumanasinghe, V.A., Bentota, A.P. & Abeysiriwardena, S. de Z. (2015) Performance of different breeding methods in cowpea (<em>Vigna unguiculata</em> (L). Walp) improvement programmes. <em>Tropical Agricultural Research</em> 26: 294–302. https://doi.org/10.4038/tar.v26i2.8093</p>
<p>Misra, J.K., Gergon, E.B. & Mew, T.W. (1995) Storage fungi and seed health of rice: A study in the Philippines. <em>Mycopathologia</em> 131: 13–24. https://doi.org/10.1007/BF01103899</p>
<p>Nawaz, U., Gul, Z. & Hayat, A. (2019) Identification of Seed Borne Mycoflora of Economically Important Vegetables of District Abbottabad and their Effect on Seed Germination and Seedling Vigor. <em>Journal of Botanical Sciences</em> 8: 7–16.</p>
<p>Oliveri, C., Torta, L. & Catara, V. (2008) A polyphasic approach to the identification of ochratoxin A-producing black <em>Aspergillus</em> isolates from vineyards in Sicily. <em>International</em> <em>Journal of Food Microbiology</em> 127: 147–154. https://doi.org/10.1016/j.ijfoodmicro.2008.06.021</p>
<p>Pak, D., You, M.P., Lanoiselet, V. & Barbetti, M.J. (2017) Reservoir of cultivated rice pathogens in wild rice in Australia. <em>European Journal of Plant Pathology</em> 147: 295–311. https://doi.org/10.1007/s10658-016-1002-y</p>
<p>Parashar, R., Rizvi, G. & Sinha, P. (2019) Seed mycoflora of some pulses collected from Bundelkhand region. <em>Journal of Pharmacognosy and Phytochemistry</em> 8: 1981–1985.</p>
<p>Pradhan, S., Lakpale, N. & Tiwari, P. (2017) Effect of Seed Treatment and Seed Borne Mycoflora on Vigour of Mungbean [<em>Vigna radiata</em> (L.) Wilczek] Grown in AgroClimatic Zones of Chhattisgarh, India. <em>International Journal of Current Microbiology and Applied Sciences</em> 6: 1946–1954. https://doi.org/10.20546/ijcmas.2017.611.231</p>
<p>Rehman, U., Irshad, G., Khan, H.M. & Ghuffar, S. (2018) Isolation, Identification and Control of mycoflora associated with paddy. <em>International Journal of Biology and Biotechnology</em> 15: 115–123.</p>
<p>Saleem, A. & Ebrahim, M.K.H. (2014) Production of amylase by fungi isolated from legume seeds collected in Almadinah Almunawwarah, Saudi Arabia. <em>Journal of Taibah University for Science</em> 8: 90–97. https://doi.org/10.1016/j.jtusci.2013.09.002</p>
<p>Tekpinar, A.D. & Kalmer, A. (2019) Utility of various molecular markers in fungal identification and phylogeny. <em>Nova Hedwigia</em> 109: 187–224. https://doi.org/10.1127/nova_hedwigia/2019/0528</p>
<p>Trabelsi, H., Neji, S., Hadrich, I., Khemakhem, N., Sellami, H., Makni, F. & Ayadi, A. (2019) Contribution of the internal transcribed spacer regions to the detection and identification of human fungal pathogens. <em>Current Research in Translational Medicine</em> 67: 100–106. https://doi.org/10.1016/j.retram.2019.04.001</p>
<p>Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., Al-Hatmi, A., Groenewald, J.Z., Cardinali, G., Houbraken, J., Boekhout, T., Crous, P.W., Robert, V. & Verkley, G.J.M. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. <em>Studies in Mycology</em> 92: 135–154. https://doi.org/10.1016/j.simyco.2018.05.001</p>
<p>White, T.J., Bruns, T.D., Lee, S & Taylor, J.W. (1990) Amplification and direct sequencing of fungal ribosomal RNA for phylogenetics. <em>In:</em> Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) <em>PCR protocols: a guide to methods and applications</em>. Academic, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1</p>
<p>Yang, S., Lee, J., Kwak, J., Kim, K., Seo, M. & Lee, Y. (2011) Fungi associated with the traditional starter cultures used for rice wine in Korea. <em>Journal of the Korean society for applied biological chemistry </em>54: 933–943. https://doi.org/10.1007/BF03253183</p>
<p>Zhang, Y.Y., Yu, Y., Wang, K., Li, M., Xu, D.S. & Zhao, J. (2016) First report of sunflower charcoal rot caused by Macrophomina phaseolina in Jilin and Inner Mongolia, China. <em>Plant Disease</em> 100: 1494–1494. https://doi.org/10.1094/PDIS-10-15-1152-PDN</p>
<p>DOA (2020) <em>National Plant Quarantine Service - Home</em>. Available from: https://www.doa.gov.lk/SCPPC/NPQS/index.php (accessed 15 June 2020)</p>
<p>CBSL (2020) <em>Annual Report 2019 | Central Bank of Sri Lanka</em>. Available from: https://www.cbsl.gov.lk/en/publications/economic-and-financial-reports/annual-reports/annual-report-2019 (accessed 16 June 2020)</p>