Abstract
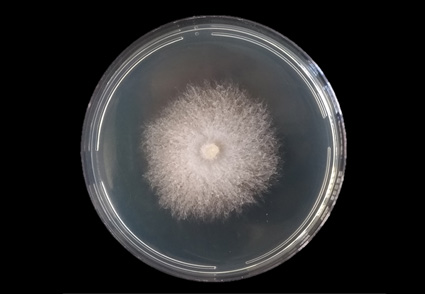
Species from Ceratobasidium are known as orchid mycorrhizal, plant pathogens or saprophytic. In mycorrhizal association with orchids, Ceratobasidium can establish a highly specific interaction. Gomesa recurva is an epiphytic orchid occurring in the Brazilian Atlantic Forest. In the present study mycorrhizal fungi were isolated from roots of G. recurva and its phylogenetic positions was investigated. A total of nine isolates were obtained directly from pelotons and submitted to phylogenetic analysis based on alignments of sequences of the internal transcribed spacer (ITS) of the nuclear ribosomal DNA. Phylogenetic analyzes, along with morphological data, revealed that isolates are distinct from all known Ceratobasidium species and a new species, namely Ceratobasidium gomesae sp. nov., is proposed. Given the dependence on mycorrhizae for germination and protocorm development, knowledge about orchid symbionts is an important factor in managing the conservation of these plants, and future studies may investigate the ability of C. gomesae to promote seed germination.
References
<p>Ceresini, P.C., Sousa, E.C., Zala, M., Furtado, E.L. & Souza, N.L. (2012) Evidence that the <em>Ceratobasidium</em>-like white-thread blight and black rot fungal pathogens from persimmon and tea crops in the Brazilian Atlantic Forest agroecosystem are two distinct phylospecies. <em>Genetics and Molecular Biology </em>35 (2): 480–497. https://doi.org/10.1590/S1415-47572012005000032</p>
<p>Costa, R.C., Verzignassi, J.R., Poltronieri, T.P.S., Poltronieri, L.S. & Monteiro, L.C. (2013) Novos hospedeiros de <em>Ceratobasidium</em> <em>ochroleucum</em>, agente causal da queima-do-fio, no Pará. <em>Summa Phytopathologica</em> 39: 62. https://doi.org/10.1590/S0100-54052013000100011</p>
<p>Dearnaley, J.D. (2007) Further advances in orchid mycorrhizal research. <em>Mycorrhiza </em>17 (6): 475–486. https://doi.org/10.1007/s00572-007-0138-1</p>
<p>Ferreira, B.W., Nóbrega, T.F. & Barreto, R.W. (2021) Reinstating <em>Ceratobasidium lantanae-camarae</em>: the white thread blight fungus on the pantropical weed <em>Lantana camara</em>. <em>Australasian Plant Pathology</em> 50: 545–557. https://doi.org/10.1007/s13313-021-00790-4</p>
<p>Freitas, E.F.S., Silva, M., Cruz, E.S., Mangaravite, E., Bocayuva, M.F., Veloso, T.G.R., Selosse, M.A. & Kasuya, M.C.M. (2020) Diversity of mycorrhizal <em>Tulasnella</em> associated with epiphytic and rupicolous orchids from the Brazilian Atlantic Forest, including four new species. <em>Scientific Reports </em>10 (1): 1–14. https://doi.org/10.1038/s41598-020-63885-w</p>
<p>Gao, Y., Zhao, Z., Li, J., Liu, N., Jacquemyn, H., Guo, S. & Xing, X. (2020) Do fungal associates of co-occurring orchids promote seed germination of the widespread orchid species <em>Gymnadenia conopsea</em>? <em>Mycorrhiza</em> 30: 221–228. https://doi.org/10.1007/s00572-020-00943-1</p>
<p>Govaerts, R., Bernet, P., Kratochvil, K., Gerlach, G., Carr, G., Alrich, P., Pridgeon, A.M., Pfahl, J., Campacci, M.A., Holland Baptista, D., Tigges, H., Shaw, J., Cribb, P.J., George, A., Kreuz, K. & Wood, J. (2021) World Checklist of Orchidaceae. Facilitated by the Royal Botanic Gardens, Kew. Available from: http://wcsp.science.kew.org/ (accessed September 2021)</p>
<p>Gónzalez, D., Rodriguez-Carres, M., Boekhout, T., Stalpers, J., Kuramae, E.E., Nakatani, A.K., Vilgalys, R. & Cubeta, M.A. (2016) Phylogenetic relationships of <em>Rhizoctonia</em> fungi within the Cantharellales. <em>Fungal Biology</em> 120 (4): 603–619. https://doi.org/10.1016/j.funbio.2016.01.012</p>
<p>Graham, R.R. & Dearnaley, J.D.W. (2012) The rare Australian epiphytic orchid <em>Sarcochilus</em> <em>weinthalii</em> associates with a single species of <em>Ceratobasidium</em>. <em>Fungal Diversity</em> 54 (1): 31–37. https://doi.org/10.1007/s13225-011-0106-0</p>
<p>IUCN (2021) The IUCN Red List of Threatened Species. Version 2021-2. Available from: https://www.iucnredlist.org (accessed 5 November 2021)</p>
<p>Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. <em>Molecular Biology and Evolution</em> 30 (4): 772–780. https://doi.org/10.1093/molbev/mst010</p>
<p>Kumar, S., Stecher, G. & Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. <em>Molecular Biology and Evolution</em> 33: 1870–1874. https://doi.org/10.1093/molbev/msw054</p>
<p>Macedo, D.M., Pereira, O.L., Hora Júnior, B.T., WEIR, B.S. & Barreto, R.W. (2016) Mycobiota of the weed <em>Tradescantia fluminensis</em> in its native range in Brazil with particular reference to classical biological control. <em>Australasian Plant Pathology</em> 45: 45–56. https://doi.org/10.1007/s13313-015-0388-x</p>
<p>Meinhardt, L.W., Bellato, C.D.M. & Tsai, S.M. (2001) SYBR® Green I used to evaluate the nuclei number of fungal mycelia. <em>Biotechniques</em> 31: 42–46. https://doi.org/10.2144/01311bm06</p>
<p>Melo, M.P., Matos, K.S., Moreira, S.I., Silva, F.F., Conceição, G.H., Nechet, K.L., Halfeld-Vieira, B.A., Bezerra Júnior, J.E.A., Ventura, J.A., Costa, H., Furtado, E.L., Alves, E. & Ceresini, P.C. (2018) Two new <em>Ceratobasidium</em> species causing white thread blight on tropical plants in Brazil. <em>Tropical Plant Pathology</em> 43 (6): 559–571. https://doi.org/10.1007/s40858-018-0237-x</p>
<p>Mendes, M.A.S. & Urben, A.F. (2021) Fungos relatados em plantas no Brasil, Laboratório de Quarentena Vegetal. Brasília, DF: Embrapa Recursos Genéticos e Biotecnologia. Available from: http://pragawall.cenargen.embrapa.br/aiqweb/michtml/fgbanco01.asp (accessed 22 August 2021)</p>
<p>Meng, Y.Y., Zhang, W.L., Selosse, M.A. & Gao, J.Y. (2019) Are fungi from adult orchid roots the best symbionts at germination? A case study. <em>Mycorrhiza</em> 29: 541–547. https://doi.org/10.1007/s00572-019-00907-0</p>
<p>Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. <em>Gateway Computing Environments Workshop: </em>1–8. https://doi.org/10.1109/GCE.2010.5676129</p>
<p>Neto, L.M., Barros, F., Vinhos, F., Furtado, S.G., Judice, D.M., Fernandez, E.P., Sfair, J.C., Barros, F.S.M., Prieto, P.V., Kutschenko, D.C., Moraes, M.A., Zanata, M.R.V. & Filho, L.A.F. dos S. (2013) Orchidaceae. <em>In:</em> Martinelli, G. & Moraes, M.A. (Eds.) <em>Livro Vermelho da Flora do Brasil</em>. Rio de Janeiro, pp.749–818.</p>
<p>Nylander, J.A.A. (2004) MrModeltest v2. Evolutionary Biology Centre, Uppsala University, Sweden. Available from: https://github.com/nylander/MrModeltest2 (accessed 5 February 2020)</p>
<p>Otero, J.T., Ackerman, J.D. & Bayman, P. (2002) Diversity and host specificity of endophytic <em>Rhizoctonia</em>-like fungi from tropical orchids. <em>American Journal of Botany</em> 89: 1852–1858. https://doi.org/10.3732/ajb.89.11.1852</p>
<p>Otero, J.T., Ackerman, J.D. & Bayman, P. (2004) Differences in mycorrhizal preferences between two tropical orchids. <em>Molecular Ecology</em> 13: 2393–2404. https://doi.org/10.1111/j.1365-294X.2004.02223.x</p>
<p>Otero, J.T., Flanagan, N.S., Herre, E.A., Ackerman, J.D. & Bayman, P. (2007) Widespread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid <em>Ionopsis utricularioides</em> (Orchidaceae). <em>American Journal of Botany</em> 94 (12): 1944–1950. https://doi.org/10.3732/ajb.94.12.1944</p>
<p>Pereira, O.L., Rollemberg, C.L., Borges, A.C., Kasuya, M.C.M. & Matsuoka, K. (2003) <em>Epulorhiza</em> <em>epiphytica</em> sp. nov. isolated from mycorrhizal roots of epiphytic orchids in Brazil. <em>Mycoscience</em> 44 (2): 153–155. https://doi.org/10.1007/S10267-002-0087-7</p>
<p>Pereira, O.L., Kasuya, M.C.M., Borges, A.C. & Araújo, E.F. (2005) Morphological and molecular characterization of mycorrhizal fungi isolated from neotropical orchids in Brazil. <em>Canadian Journal Botany</em> 83: 54–65. https://doi.org/10.1139/b04-151</p>
<p> Pereira, M.C., Coelho, I.S., Valadares, R.B.S., Oliveira, S.F., Bocayuva, M., Pereira, O.L., Araújo, E.F. & Kasuya, M.C.M. (2014) Morphological and molecular characterization of <em>Tulasnella</em> spp. fungi isolated from the roots of <em>Epidendrum secundum</em>, a widespread Brazilian orchid. <em>Symbiosis</em> 62: 111–121. https://doi.org/10.1007/s13199-014-0276-0</p>
<p>Pereira, M.C., Pereira, O.L., Costa, M.D., Rocha, R.B. & Kasuya, M.C.M. (2009) Diversidade de fungos micorrízicos <em>Epulorhiza</em> spp. isolados de <em>Epidendrum secundum</em> (Orchidaceae). <em>Revista Brasileira de Ciência do Solo</em> 33: 1187–1197. https://doi.org/10.1590/S0100-06832009000500012</p>
<p> Nogueira, R.E., Pereira, O.L., Kasuya, M.C.M., Lanna, M.C.S. & Mendonça, M.P. (2005) Fungos micorrízicos associados a orquídeas em campos rupestres na região do Quadrilátero Ferrífero, MG, Brasil. <em>Acta Botanica Brasilica</em> 19 (3): 417–424. https://doi.org/10.1590/S0102-33062005000300001</p>
<p>Rasmussen, H.N. (2002) Recent developments in the study of orchid mycorrhiza. <em>Plant and Soil</em> 244 (1–2): 149–163. https://doi.org/10.1023/A:1020246715436</p>
<p>Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Beyesian phylogenetic inference under mixed models. <em>Bioinformatics</em> 19: 1572–1574. https://doi.org/10.1093/bioinformatics/btg180</p>
<p>Rambaut, A. (2009) FigTree version 1.4.4. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 5 February 2020)</p>
<p>Rammitsu, K., Yagame, T., Yamashita, Y., Yukawa, T., Isshiki, S. & Ogura-Tsujita, Y. (2019) A leafless epiphytic orchid, <em>Taeniophyllum glandulosum</em> Blume (Orchidaceae), is specifically associated with the Ceratobasidiaceae family of basidiomycetous fungi. <em>Mycorrhiza</em> 29 (2): 159–166. https://doi.org/10.1007/s00572-019-00881-7</p>
<p>Rannala, B. & Yang, Z. (1996) Proballity distribution of molecular evolutionary trees: A new method of phylogenetic inference. <em>Journal of Molecular Evolution</em> 43: 304–311. https://doi.org/10.1007/BF02338839</p>
<p>Smith, S.E. & Read, D.J. (2008) <em>Mycorrhizal Symbiosis</em>, 3rd edn. Adelaide, Australia.</p>
<p>Thixton, H.L., Esselman, E.J., Corey, L.L. & Zettler, L.W. (2020) Further evidence of <em>Ceratobasidium</em> D.P. Rogers (Basidiomycota) serving as the ubiquitous fungal associate of <em>Platanthera leucophaea</em> (Orchidaceae) in the North American tallgrass prairie. <em>Botanical Studies</em> 61: 12. https://doi.org/10.1186/s40529-020-00289-z</p>
<p>Valadares, R.B., Pereira, M.C., Otero, J.T. & Cardoso, E.J. (2012) Narrow fungal mycorrhizal diversity in a population of the orchid <em>Coppensia</em> <em>doniana</em>. <em>Biotropica</em> 44 (1): 114–122. https://doi.org/10.1111/j.1744-7429.2011.00769.x</p>
<p>Warcup, J.H. & Talbot, P.H.B. (1980) Perfect states of Rhizoctonias associated with orchids. <em>New Phytologist</em> 66 (4): 631–641. https://doi.org/10.1111/j.1469-8137.1980.tb00787.x</p>
<p>Weiß, M., Waller, F., Zuccano, A. & Selosse, M.A. (2016) Sebacinales – one thousand and one interactions with land plants. <em>New Phytologist</em> 211: 20–40. https://doi.org/10.1111/nph.13977</p>
<p>White, T.J., Bruns, T.D., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. <em>In:</em> Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) <em>PCR protocols: a guide to methods and applications</em>. Academic, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1</p>
<p>Zettler, L.W., Piskin, K.A., Stewart, S.L., Hartsock, J.J., Bowles, M.L. & Bell, T.J. (2005) Protocorm mycobionts of the Federally threatened Eastern Prairie Fringed Orchid, <em>Platanthera leucophaea</em> (Nutt.) Lindl., and a technique to prompt leaf elongation in seedlings. <em>Studies in Mycology</em> 53: 163–171. https://doi.org/10.3114/sim.53.1.163</p>