Abstract
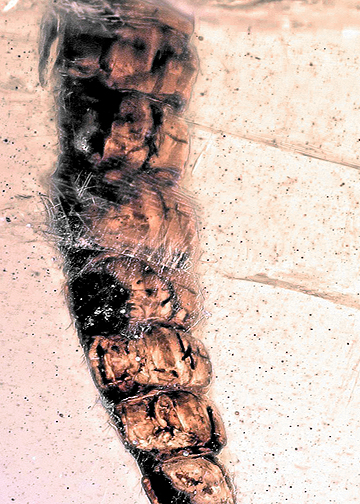
The evolutionary success of Insecta, more precisely of its ingroup Holometabola, has partly been explained by their ontogeny, with larvae and adults differing in their morphology and ecology. This differentiation occurs in large and well-known groups such as beetles, butterflies and bees, but also in the relatively species-poor group of snakeflies (Raphidioptera). Despite the rather small number of species, snakeflies are evolutionarily very significant as they were part of the early diversification of Holometabola and still exhibit several plesiomorphic traits retained from the ground pattern of the latter, for example, a mobile pupa. Furthermore, during development, some snakeflies show a mixture of larval and pupal, sometimes even of adult characters, a phenomenon called metathetely. We here report a 100 million-year-old fossil snakefly larva from Myanmar amber with possible characters reminiscent of metathetely. Different dimensions of the body were measured in the specimen and other snakefly larvae and pupae, and ratios were calculated and compared among the larvae. The new fossil shows similarities to extant pupae in the larger length of the prothorax, similarities to modern adults in the small width of the prothorax, but also similarities to other fossil snakefly larvae such as the undivided tarsus and the antenna being subdivided into only five elements. Such a mixture of characters from different developmental stages points to a less pronounced metamorphosis in fossil snakeflies than in extant ones. Similar ontogenetic patterns, with a more gradual development in earlier representatives evolving into a more pronounced metamorphosis in modern representatives, are also known in other groups of Euarthropoda and point to heterochronic events in the evolution of these lineages.
References
- Aspöck, U. & Aspöck, H. (2007) Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia, 20, 451–516.
- Aspöck, H., Abbt, V., Aspöck, U. & Gruppe, A. (2018) The phenomenon of metathetely, formerly known as prothetely, in Raphidioptera (Insecta: Holometabola: Neuropterida). Entomologia Generalis, 37(3–4), 197–230. https://doi.org/10.1127/entomologia/2018/0646
- Aspöck, H., Aspöck, U. & Gruppe, A. (2019) Metathetely and its implications for the distribution of Raphidioptera (Insecta, Holometabola: Neuropterida). In: Weihrauch, F., Frank, O., Gruppe, A., Jepson, J.E., Kirschey, L. & Ohl, M. (Eds), Proceedings of the XIII International Symposium of Neuropterology, 17–22 June 2018, Laufen/Salzach, Bavaria, Germany. Osmylus Scientific Publishers, Wolnzach, Germany pp. 79–93. https://doi.org/10.5281/zenodo.3569383
- Crespo-Pérez, V., Kazakou, E., Roubik, D.W. & Cárdenas, R.E. (2020) The importance of insects on land and in water: a tropical view. Current Opinion in Insect Science, 40, 31–38. https://doi.org/10.1016/j.cois.2020.05.016
- Cruickshank, R.D. & Ko, K. (2003) Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences, 21, 441–455. https://doi.org/10.1016/S1367-9120(02)00044-5
- Gosik, R. (2006) Description of the larva and the pupa of Bagous nodulosus Gyllenhal in Schoenherr, 1836 (Coleoptera: Curculionidae), with comments on its biology. Baltic Journal of Coleopterology, 6, 143–153.
- Gosik, R. (2009) Description of the mature larva and pupa of Bagous lutulentus (Gyllenhal), with comments on its biology (Coleoptera: Curculionidae). Genus, 20 (1), 125–135.
- Gosik, R. (2010) Morphology of the mature larva and pupa of Rhinusa bipustulata (Rossi, 1792) (Coleoptera: Curculionidae) with some remarks on its biology. Baltic Journal of Coleopterology, 10 (2), 185–194.
- Gosik, R. & Sprick, P. (2013) Morphology and identification of the pupae of several species of soil-dwelling broad-nosed weevils from Central Europe (Coleoptera, Curculionidae, Entiminae). Zootaxa, 3731 (4), 445–472. https://doi.org/10.11646/zootaxa.3731.4.2
- Haug, C., Mayer, G., Kutschera, V., Waloszek, D., Maas, A. & Haug, J.T. (2011) Imaging and documenting gammarideans. International Journal of Zoology, 2011, 380829. https://doi.org/10.1155/2011/380829
- Haug, C., Shannon, K.R., Nyborg, T. & Vega, F.J. (2013b) Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Boletín de la Sociedad Geológica Mexicana, 65, 273–284. https://doi.org/10.18268/BSGM2013v65n2a9
- Haug, J.T. (2019) Categories of developmental biology: Examples of ambiguities and how to deal with them. In: Fusco, G. (Ed.), Perspectives on evolutionary and developmental biology. Essays for Alessandro Minelli. Festschrift 2. Padova University Press, Padova, pp. 93–102.
- Haug, J.T. (2020a) Why the term “larva” is ambiguous, or what makes a larva? Acta Zoologica, 101, 167–188. https://doi.org/10.1111/azo.12283
- Haug, J.T. (2020b) Metamorphosis in crustaceans. In: Cothran, R.D. & Thiel, M. (Eds), Developmental biology and larval ecology. The natural history of the Crustacea. Volume 7. Oxford University Press, Oxford, pp. 254–283.
- Haug, J.T. & Haug, C. (2013) An unusual fossil larva, the ontogeny of achelatan lobsters, and the evolution of metamorphosis. Bulletin of Geosciences, 88, 195–206. https://doi.org/10.3140/bull.geosci.1374
- Haug, J.T. & Haug, C. (2015) “Crustacea”: comparative aspects of larval development. In: Wanninger, A. (Ed.), Evolutionary developmental biology of invertebrates 4. Ecdysozoa II: “Crustacea”. Springer, Wien, pp. 1–37.
- Haug, J.T. & Haug, C. (2016) “Intermetamorphic” developmental stages in 150 million-year-old achelatan lobsters—The case of the species tenera Oppel, 1862. Arthropod Structure & Development, 45, 108–121. https://doi.org/10.1016/j.asd.2015.10.001
- Haug, J.T., Maas, A. & Waloszek, D. (2010a) †Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 100, 311–350. https://doi.org/10.1017/S1755691010008145
- Haug, J.T., Waloszek, D., Haug, C. & Maas, A. (2010b) High-level phylogenetic analysis using developmental sequences: the Cambrian †Martinssonia elongata, †Musacaris gerdgeyeri gen. et sp. nov. and their position in early crustacean evolution. Arthropod Structure & Development, 39, 154–173. https://doi.org/10.1016/j.asd.2010.01.005
- Haug, J.T., Müller, C.H.G. & Sombke, A. (2013a) A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity and Evolution, 13, 425–432. https://doi.org/10.1007/s13127-013-0129-3
- Haug, J.T., Audo, D., Charbonnier, S. & Haug, C. (2013c) Diversity of developmental patterns in achelate lobsters—today and in the Mesozoic. Development Genes and Evolution, 223, 363–373. https://doi.org/10.1007/s00427-013-0452-x
- Haug, J.T., Hädicke, C.W., Haug, C. & Hörnig, M.K. (2015) A possible hatchling of a jumping bristletail in 50 million years old amber. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 278, 191–199. https://doi.org/10.1127/njgpa/2015/0523
- Haug, J.T., Haug, C. & Garwood, R. (2016) Evolution of insect wings and development—new details from Palaeozoic nymphs. Biological Reviews, 91, 53–69. https://doi.org/10.1111/brv.12159
- Haug, J.T., Müller, P. & Haug, C. (2018) The ride of the parasite: A 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters, 4, 31. https://doi.org/10.1186/s40851-018-0116-9
- Haug, J. T., Müller, P. & Haug, C. (2019a) A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zoological Letters, 5, 29. https://doi.org/10.1186/s40851-019-0144-0
- Haug, J.T., Müller, P. & Haug, C. (2019b) A 100-million-year old slim insectan predator with massive venom-injecting stylets—a new type of neuropteran larva from Burmese amber. Bulletin of Geosciences, 94, 431–440. https://doi.org/10.3140/bull.geosci.1753
- Haug, J.T., Haug, C. & Schweigert, G. (2019c) The oldest “intermetamorphic” larva of an achelatan lobster from the Lower Jurassic Posidonia Shale, South Germany. Acta Palaeontologica Polonica, 64, 685–692. https://doi.org/10.4202/app.00627.2019
- Haug, J. T., Müller, P. & Haug, C. (2020) A 100 million-year-old snake-fly larva with an unusually large antenna. Bulletin of Geosciences, 95, 167–177. https://doi.org/10.3140/bull.geosci.1757
- Haug, J.T., Baranov, V., Müller, P. & Haug, C. (2021) New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Scientific Reports, 11, 20432. https://doi.org/10.1038/s41598-021-99480-w
- Haug, J.T., Engel, M.S., Mendes dos Santos, P., Haug, G.T., Müller, P. & Haug, C. (2022) Declining morphological diversity in snakefly larvae during last 100 million years. PalZ, 96, 749–780. https://doi.org/10.1007/s12542-022-00609-7
- Jankielsohn A. (2018) The importance of insects in agricultural ecosystems. Advances in Entomology, 6 (2), 62–73. https://doi.org/10.4236/ae.2018.62006
- Jindra, M. (2019) Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Philosophical Transactions of the Royal Society B, 374, 20190064. https://doi.org/10.1098/rstb.2019.0064
- May, B.M. (1987) Immature stages of Curculionidae (Coleoptera): the larva and pupa of Karocolens pittospori (Molytinae). New Zealand Entomologist, 9 (1), 29–34. https://doi.org/10.1080/00779962.1987.9722489
- Oseto, C.Y. & Braness, G.A. (1979) Description of the larva and pupa of Smicronyx fulvus LeConte (Coleoptera: Curculionidae). Journal of the Kansas Entomological Society, 52, 103–108.
- Pruthi, H.S. (1927) Prothetely in insects. Nature, 119 (2993), 391–392. https://doi.org/10.1038/119391a0
- Saltin, B.D., Haug, C. & Haug, J.T. (2016) How metamorphic is holometabolous development? Using microscopical methods to look inside the scorpionfly (Panorpa) pupa. Spixiana, 39, 105–118.
- Shi, G.H., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M.C., Lei, W.Y., Li, Q.L. & Li, X.H. (2012) Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37, 155–163. https://doi.org/10.1016/j.cretres.2012.03.014
- Sousa, W.O.D., Rosado-Neto, G.H., Moreira, M.A. & Zarbin, P.H. (2004) Description of the larva and pupa of the papaw borer weevil Pseudopiazurus papayanus (Marshall) (Coleoptera, Curculionidae, Piazurini). Revista Brasileira de Entomologia, 48, 331–334. https://doi.org/10.1590/S0085-56262004000300007
- Stejskal, R., Trnka, F. & Skuhrovec, J. (2014) Biology and morphology of immature stages of Coniocleonus nigrosuturatus (Coleoptera: Curculionidae: Lixinae). Acta Entomologica Musei Nationalis Pragae, 54 (1), 337–354.
- Suter, G.W. & Cormier, S.M. (2015) Why care about aquatic in-sects: uses, benefits, and services. Integrated Environmental Assessment and Management, 11 (2), 188–194. https://doi.org/10.1002/ieam.1600
- Truman, J.W. & Riddiford, L.M. (2019) The evolution of insect metamorphosis: a developmental and endocrine view. Philosophical Transactions of the Royal Society B, 374 (1783), 20190070. https://doi.org/10.1098/rstb.2019.0070
- Wachmann E. & Saure C. (1997) Netzflügler, Schlamm- und Kamelhalsfliegen. Naturbuch-Verlag, Augsburg, 159 pp.
- Wagner, D.L., Grames, E.M., Forister, M.L., Berenbaum, M.R. & Stopak, D. (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proceedings of the National Academy of Sciences, 118, e2023989118. https://doi.org/10.1073/pnas.2023989118
- Wermelinger, B. (2021) Forest insects in Europe: diversity, functions and importance. CRC Press, Boca Raton, 365 pp. https://doi.org/10.1201/9781003186465
- Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F., Zhang, H., Wang, B. & Dilcher, D. (2019) An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences of the USA, 116, 11345–11350. https://doi.org/10.1073/pnas.1821292116
- Zhang, X.G., Siveter, D.J., Waloszek, D. & Maas, A. (2007) An epipodite-bearing crown-group crustacean from the Lower Cambrian. Nature, 449 (7162), 595–598. https://doi.org/10.1038/nature06138
- Zhao, C.J., Ang, Y.C., Wang, M.Q., Gao, C.X., Zhang, K.Y., Tang, C.F., Liu, X.Y., Li, M., Yang, D. & Meier, R. (2020) Contribution to understanding the evolution of holometaboly: transformation of internal head structures during the metamorphosis in the green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). BMC Evolutionary Biology, 20, 79. https://doi.org/10.1186/s12862-020-01643-2
- Zimmerman, D., Randolf, S. & Aspöck, U. (2019) From chewing to sucking via phylogeny—from sucking to chewing via ontogeny: mouthparts of Neuroptera. In: Krenn, H.W. (Ed.), Insect mouthparts: form, function, development and performance. Springer, Cham, pp. 361–385. https://doi.org/10.1007/978-3-030-29654-4_11