Abstract
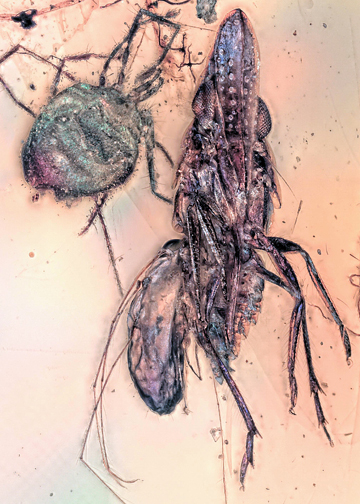
The fossilization of organisms in amber displaying the behaviour in which they were engaged when becoming entrapped in tree resin is known as “frozen behaviour” (Arillo, 2007). Findings of frozen behaviour are rare and can uncover valuable information on how food webs were structured in the past. One of the most reported interactions between organisms in amber is the case of parasitengonan mites still attached to their hosts (De Baets et al., 2021). Parasitengona is an ingroup of Trombidiformes characterised by a complex life cycle (Krantz & Walter, 2009) in which the active post-larval forms (deutonymph and adult) are predatory, while the six-legged heteromorphic larva (for challenges of the term, see Haug, 2020) is parasitic on euarthropodan and vertebrate hosts (Mąkol et al., 2012). Given the firm grip of most parasitengonan mites to their hosts (e.g., Åbro, 1988), these mites are often found in amber still attached to them (De Baets et al., 2021). Among Parasitengona, there is an ingroup characterised by their extremely long legs (Wohltmann, 2000), Erythraeoidea, or long-legged velvet mites. Erythraeoidean larvae parasitise several euarthropodan groups, with few exceptions (Stroiński et al., 2013). Most known hosts from the fossil record are dipterans (as reviewed by Arillo et al., 2018; Arce et al., 2024), with a few reports on other insects: a moth (Poinar et al., 1991), a spider and a booklouse (Weitschat & Wichard 1998), and a cicada (Poinar et al., 2012). Here we report a piece of Kachin (Myanmar) amber of ca. 100 Mya with a mite attached to a planthopper. We further discuss its implications for the host-parasite relationship of Erythraeoidea.
References
- Åbro, A. (1988) The mode of attachment of mite larvae (Leptus spp.) to harvestmen (Opiliones). Journal of Natural History, 22 (1), 123–130. https://doi.org/10.1080/00222938800770091
- Amaral, A.P., Stotzem, L., Haug, G.T., Haug, C. & Haug, J.T. (2024) Immature planthoppers had longer mouthparts 100 million years ago as exemplified by quantitative morphology (Hemiptera, Fulgoromorpha). Spixiana, 46 (2), 201–226.
- Arce, S.I., Haug, C., Haug, J.T. & Amaral, A.P. (2024) Driven apart: fossil parasitic long-legged velvet mite larvae on gall midges represent a long lost parasitic association between mites and dipterans. Palaeoentomology, 7 (2), 254–264. https://doi.org/10.11646/palaeoentomology.7.2.9
- Arillo, A. (2007) Paleoethology: Fossilized behaviours in amber. Geologica Acta, 5 (2), 159–166. https://doi.org/10.1344/105.000000301
- Arillo, A., Blagoderov, V. & Peñalver, E. (2018) Early Cretaceous parasitism in amber: A new species of Burmazelmira fly (Diptera: Archizelmiridae) parasitized by a Leptus sp. mite (Acari, Erythraeidae). Cretaceous Research, 86, 24–32. https://doi.org/10.1016/j.cretres.2018.02.006
- De Baets, K., Huntley, J., Klompmaker, A., Schiffbauer, J. & Muscente, A. (2021) The fossil record of parasitism: its extent and taphonomic constraints. In: De Baets, K. & Huntley, J.W. (Eds), The evolution and fossil record of parasitism: coevolution and paleoparasitological techniques. Topics in Geobiology 50. Springer, Cham, Switzerland, 1–50. https://doi.org/10.1007/978-3-030-52233-9_1
- Emeljanov, A.F. & Shcherbakov, D.E. (2018) The longest-nosed Mesozoic Fulgoroidea (Homoptera): a new family from mid-Cretaceous Burmese amber. Far Eastern Entomologist, 354, 1–14. https://doi.org/10.25221/fee.354.1
- Haug, J.T. (2020) Why the term “larva” is ambiguous, or what makes a larva? Acta Zoologica, 101, 167–188. https://doi.org/10.1111/azo.12283
- Krantz, G.W. & Walter, D.E. (2009) A manual of acarology. 3rd ed. Texas Tech University Press, Lubbock, 807 pp.
- LaMunyon, C. & Eisner, T. (1990) Effect of mite infestation on the anti-predator defenses of an insect. Psyche: A Journal of Entomology, 97, 31–41. https://doi.org/10.1155/1990/70973
- Mąkol, J., Kłosińska, A. & Łaydanowicz, J. (2012) Host–parasite interactions within terrestrial Parasitengona (Acari, Trombidiformes, Prostigmata). International Journal of Acarology, 38 (1), 18–22. https://doi.org/10.1080/01647954.2011.583276
- Mąkol, J., Moniuszko, H., Świerczewski, D. & Stroiński, A. (2014) Planthopper (Hemiptera: Flatidae) parasitized by larval erythraeid mite (Trombidiformes: Erythraeidae)—A description of two new species from western Madagascar. Journal of Insect Science, 14 (1), 194. https://doi.org/10.1093/jisesa/ieu056
- Poinar, G.O., Treat, A.E. & Southcott, R.V. (1991) Mite parasitism of moths: Examples of paleosymbiosis in Dominican amber. Experientia, 47 (2), 210–212. https://doi.org/10.1007/BF01945430
- Poinar, G., Kritsky, G. & Brown, A. (2012) Minyscapheus dominicanus n. gen., n. sp. (Hemiptera: Cicadidae), a fossil cicada in Dominican amber. Historical Biology, 24 (3), 329–333. https://doi.org/10.1080/08912963.2011.635791
- Shi, G.H., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M.C., Lei, W.Y., Li, Q.L. & Li, X.H. (2012) Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37, 155–163. https://doi.org/10.1016/j.cretres.2012.03.014
- Southcott, R. V. (1961) Studies on the systematics and biology of the Erythraeoidea (Acarina), with critical revision of the genera and subfamilies. Australian Journal of Zoology, 9 (3), 367–610. https://doi.org/10.1071/ZO9610367
- Stroiński, A., Felska, M. & Mąkol, J. (2013) A review of host-parasite associations between terrestrial Parasitengona (Actinotrichida: Prostigmata) and bugs (Hemiptera). Annales Zoologici, 63 (2), 195–221. https://doi.org/10.3161/000345413X669522
- Torrico-Bazoberry, D., Pinto, C.F., Davyt-Colo, J. & Niemeyer, H.M. (2020) Response to selected ecological parameters by Leptus hringuri Haitlinger, 2000 larvae (Trombidiformes: Erythraeidae) parasitizing treehoppers (Hemiptera: Membracidae) from Bolivia on two host-plant species. International Journal of Acarology, 46 (3), 174–179. https://doi.org/10.1080/01647954.2020.1751280
- Weitschat, W. & Wichard, W. (1998) Atlas der Pflanzen und Tiere im Baltischen Bernstein. Verlag Dr Friedrich Pfeil, München, 256 pp.
- Wohltmann, A. (2000) The evolution of life histories in Parasitengona (Acari: Prostigmata). Acarologia, 41 (1–2), 145–204.
- Xu, S.Y., Yi, T.C., Guo, J.J. & Jin, D.C. (2020) Four new species of larval Callidosomatinae (Acari: Prostigmata: Erythraeidae) and a newly recorded genus Iguatonia from China with notes on generic concept. Systematic and Applied Acarology, 25 (2), 285–326. https://doi.org/10.11158/saa.25.2.9