Abstract
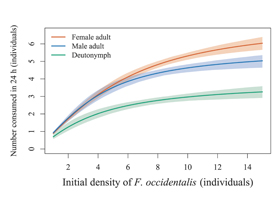
Western flower thrips, Frankliniella occidentalis, remains difficult to manage in crops because their non-feeding pupal stage is largely concealed in the soil, making it unreachable by foliar insecticides and canopy-dwelling natural enemies. The soil-dwelling laelapid mite Stratiolaelaps scimitus is widely used against edaphic pests, but its performance on thrips pupae relative to factitious prey, and the contribution of different life stages to thrips suppression, are still poorly quantified. We first compared no-choice predation and fecundity of adult females offered either thrips pupae or the dried fruit mite Carpoglyphus lactis (male adults), with a starvation treatment as control. S. scimitus females readily attacked both prey types and produced significantly more eggs when food was available than under starvation, but 48 h oviposition did not differ significantly between the two diets, indicating that thrips pupae and C. lactis provided comparable short-term nutritional support. Next, we quantified the functional responses of S. scimitus deutonymphs, adult males and adult females, to thrips pupae across six prey densities (1, 3, 5, 8, 12 or 15 pupae per arena) over 24 h. All tested stages of S. scimitus exhibited a Type II functional response. Adult females had the shortest handling time and highest asymptotic consumption (≈7.6 pupae per day), adult males were intermediate (≈5.8 pupae day⁻¹), and deutonymphs the lowest (≈3.9 pupae day⁻¹). These results confirm S. scimitus as an effective predator of thrips pupae, with mixed-stage populations contributing to pupal mortality, and support current mass-rearing practices on factitious prey for subsequent deployment against this pest in crops.
References
- Albendín G., Del Castillo García M. & Molina J. M. 2015. Multiple natural enemies do not improve two spotted spider mite an flower western thrips control in strawberry tunnels. Chilean Journal of Agricultural Research 75(1): 63–70. https://doi.org/10.4067/S0718-58392015000100009
- Amarathunga D. C., Parry H., Grundy J. & Dorin A. 2024. A predator–prey population dynamics simulation for biological control of Frankliniella occidentalis (Western Flower Thrips) by Orius laevigatus in strawberry plants. Biological Control 188: 105409. https://doi.org/10.1016/j.biocontrol.2023.105409
- Amini P., Poorjavad N., Khajehali J. & Ardestani M. M. 2025. Life table parameters of the predatory mite Amblyseius swirskii on different diets of Rhizoglyphus robini and its functional response on Tetranychus urticae. International Journal of Tropical Insect Science 45(3): 1057–1065. https://doi.org/10.1007/s42690-025-01492-w
- Berndt O., Meyhöfer R. & Poehling H.-M. 2004a. The edaphic phase in the ontogenesis of Frankliniella occidentalis and comparison of Hypoaspis miles and Hypoaspis aculeifer as predators of soil-dwelling thrips stages. Biological Control 30(1): 17–24. https://doi.org/10.1016/j.biocontrol.2003.09.009
- Berndt O., Poehling H.‐M. & Meyhöfer R. 2004b. Predation capacity of two predatory laelapid mites on soil‐dwelling thrips stages. Entomologia Experimentalis et Applicata 112(2): 107–115. https//doi.org/10.1111/j.0013-8703.2004.00185.x
- Buitenhuis R. & Shipp J. L. 2008. Influence of plant species and plant growth stage on Frankliniella occidentalis pupation behaviour in greenhouse ornamentals. Journal of Applied Entomology 132(1): 86–88. https://doi.org/10.1111/j.1439-0418.2007.01250.x
- Castilho R. C., De Moraes G. J., Silva E. S., Freire R. A. P. & Da Eira F. C. 2009. The predatory mite Stratiolaelaps scimitus as a control agent of the fungus gnat Bradysia matogrossensis in commercial production of the mushroom Agaricus bisporus. International Journal of Pest Management 55(3): 181–185. https://doi.org/10.1080/09670870902725783
- Castro-López M. A., Ramírez-Godoy A., Martínez Osorio W. & Rueda-Ramírez D. 2021. Predation and oviposition rates of Gaeolaelaps aculeifer and Parasitus bituberosus (Acari: Laelapidae and Parasitidae) on pre-pupae/pupae of Thrips tabaci (Thysanoptera: Thripidae). Acarologia 61(2): 394–402. https://doi.org/10.24349/acarologia/20214438
- Cloyd R. A. 2019. Effects of predators on the belowground life stages (prepupae and pupae) of the western flower thrips, Frankliniella occidentali (Thripidae: Thysanoptera): A review. Advances in Entomology 07(04): 71–80. https://doi.org/10.4236/ae.2019.74006
- Dalir S., Hajiqanbar H., Fathipour Y. & Khanamani M. 2025. Effectiveness of the predatory mite Neoseiulus cucumeris on two-spotted spider mite and western flower thrips: A quantitative assessment. Bulletin of Entomological Research 115(2): 184–193. https://doi.org/10.1017/S0007485325000033
- Ebssa L., Borgemeister C. & Poehling H.-M. 2006. Simultaneous application of entomopathogenic nematodes and predatory mites to control western flower thrips Frankliniella occidentalis. Biological Control 39(1): 66–74. https://doi.org/10.1016/j.biocontrol.2006.02.005
- Funderburk J., Martini X., Freeman J., Strzyzewski I., Traczyk E., Skarlinsky T. & Adkins S. 2019. Sampling for estimating Frankliniella species Flower Thrips and Orius species predators in field experiments. Journal of Visualized Experiments (149): 59869. https://doi.org/10.3791/59869
- Gao R., Lu R., Qiu X., Wang L., Zhang K. & Wu S. 2023. Detection of putative mutation I873S in the sodium channel of Megalurothrips usitatus (Bagnall) which may be associated with pyrethroid resistance. Insects 14(4): 388. https://doi.org/10.3390/insects14040388
- Hassell M. P. & Comins H. N. 1978. Sigmoid functional responses and population stability. Theoretical Population Biology 14(1): 62–67. https://doi.org/10.1016/0040-5809(78)90004-7
- Holling C. S. 1959. Some characteristics of simple types of predation and parasitism. The Canadian Entomologist 91(7): 385–398. https://doi.org/10.4039/Ent91385-7
- Juliano S. A. 2001. Nonlinear curve fitting: predation and functional response curves. In: Design and Analysis of Ecological Experiments. (S.M. Scheiner, & J. Gurevitch, editors). Oxford University Press.
- Kay M., Elkin L.A., Higgins J. J. & Wobbrock J. O. 2014. ARTool: Aligned Rank Transform. 0.11.2.
- Knapp M., Van Houten Y., Van Baal E. & Groot T. 2018. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 58(Suppl): 72–82. https://doi.org/10.24349/acarologia/20184275
- Lan Q.-X., Wen M.-F., Lu Z.-H., Ke B.-R., Fan Q.-H. & You M.-S. Control of fungus gnats Lycoriella sp. in mushroom (Agrocybe aegerita) cultivation with predatory mites Macrocheles glaber (Acari: Macrochelidae) and Stratiolaelaps scimitus (Acari: Laelapidae). Insect Science n/a (n/a). https://doi.org/10.1111/1744-7917
- Liu W., Zhang K., Li L. & Zhang Z.-Q. 2025. What factors influence the plasticity of a facultative feeding larval predator Amblydromalus limonicus (Garman and McGregor) (Acari: Phytoseiidae)? Acarologia 65(2): 270–279. https://doi.org/10.24349/6poj-q5dq
- Mazzutti M., Bisognin R. P., Guerra D., Bohrer R. E. G., Da Silva D. M. & De Souza E. L. 2023. Biological control of fungus gnats (Bradysia matogrossensis) in tobacco seedlings. Revista Caatinga 36(2): 464–470. https://doi.org/10.1590/1983-21252023v36n223rc
- Meehan M. L., Turnbull K. F., Sinclair B. J. & Lindo Z. 2022. Predators minimize energy costs, rather than maximize energy gains under warming: Evidence from a microcosm feeding experiment. Functional Ecology 36(9): 2279–2288. https://doi.org/10.1111/1365-2435.14131
- Messelink G. J., Van Steenpaal S. E. F. & Ramakers P. M. J. 2006. Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl 51(6): 753–768. https://doi.org/10.1007/s10526-006-9013-9
- Murunde R., Wainwright H. & Turoop L. 2019. Rate of consumption of Western flower thrips pupae by the soil-dwelling mite Hypoaspis sclerotarsa (Acari: Laelapidae). Biocontrol Science and Technology 29(8): 796–803. https://doi.org/10.1080/09583157.2019.1608505
- Navarro-Campos C., Wäckers F. L. & Pekas A. 2016. Impact of factitious foods and prey on the oviposition of the predatory mites Gaeolaelaps aculeifer and Stratiolaelaps scimitus (Acari: Laelapidae). Experimental and Applied Acarology 70(1): 69–78. https://doi.org/10.1007/s10493-016-0061-2
- Nielsen M.-C., Barratt B., Thompson C., Chhagan A., Puketapu A., Horne P. & Vereijssen J. 2025. Augmentative biological control of invertebrate pests in Australasia: Experiences from down under. Biological Control 210: 105895. https://doi.org/10.1016/j.biocontrol.2025.105895
- Park J., Mostafiz M. M., Hwang H.-S., Jung D.-O. & Lee K.-Y. 2021a. Comparing the life table and population projection of Gaeolaelaps aculeifer and Stratiolaelaps scimitus (Acari: Laelapidae) based on the age-stage, two-sex life table theory. Agronomy 11(6): 1062. https://doi.org/10.3390/agronomy11061062
- Park J., Munir Mostafiz M., Hwang H.-S., Jung D.-O. & Lee K.-Y. 2021b. Comparison of the predation capacities of two soil-dwelling predatory mites, Gaeolaelaps aculeifer and Stratiolaelaps scimitus (Acari: Laelapidae), on three thrips species. Journal of Asia-Pacific Entomology 24(1): 397–401. https://doi.org/10.1016/j.aspen.2021.01.009 Pergande T. 1895. Notes on certain Thysanoptera of North America. Insect Life 7: 390–397.
- Pozzebon A., Boaria A. & Duso C. 2015. Single and combined releases of biological control agents against canopy- and soil-dwelling stages of Frankliniella occidentalis in cyclamen. BioControl 60(3): 341–350. https://doi.org/10.1007/s10526-014-9641-4
- Prado Freire A. R. & De Moraes G. J. 2007. Mass production of the predatory mite Stratiolaelaps scimitus (Womersley) (Acari: Laelapidae). Systematic and Applied Acarology 12(2): 117. https://doi.org/10.11158/saa.12.2.4
- Price B. E., Raffin C., Yun S. H., Velasco-Graham K. & Choi M.-Y. 2022. A sustainable mass rearing method for western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Florida Entomologist 105 (2). https://doi.org/10.1653/024.105.0211
- Pritchard D. W., Paterson R. A., Bovy H. C. & Barrios‐O’Neill D. 2017. FRAIR: An R package for fitting and comparing consumer functional responses. (T. Poisot, editor). Methods in Ecology and Evolution 8(11): 1528–1534. https://doi.org/10.1111/2041-210X.12784
- R Core Team. 2024. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/ (accessed on 28 December 2025)
- Reitz S. R., Gao Y., Kirk W. D. J., Hoddle M. S., Leiss K. A. & Funderburk J. E. 2020. Invasion biology, ecology, and management of western flower thrips. Annual Review of Entomology 65(1): 17–37. https://doi.org/10.1146/annurev-ento-011019-024947
- Rogers D. 1972. Random search and insect population models. The Journal of Animal Ecology 41(2): 369. https://doi.org/10.2307/3474
- Saito T. & Brownbridge M. 2018. Compatibility of foliage-dwelling predatory mites and mycoinsecticides, and their combined efficacy against western flower thrips Frankliniella occidentalis. Journal of Pest Science 91(4): 1291–1300. https://doi.org/10.1007/s10340-018-0991-z
- Shalileh S., Ogada P. A., Moualeu D. P. & Poehling H.-M. 2016. Manipulation of Frankliniella occidentalis (Thysanoptera: Thripidae) by Tomato Spotted Wilt Virus (Tospovirus) Via the host plant nutrients to enhance its transmission and spread. Environmental Entomology 45(5): 1235–1242. https://doi.org/10.1093/ee/nvw102
- Srivastava M., Funderburk J., Olson S., Demirozer O. & Reitz S. 2014. Impacts on natural enemies and competitor thrips of insecticides against the western flower thrips (Thysanoptera: Thripidae) in fruiting vegetables. Florida Entomologist 97(2): 337–348. https://doi.org/10.1653/024.097.0201
- Teulon D. A. J. & Nielsen M.-C. 2005. Distribution of western (Glasshouse strain) and intonsa flower thrips in New Zealand. New Zealand Plant Protection 58: 208–212. https://doi.org/10.30843/nzpp.2005.58.4274
- Wang J., Zhang K., Li L. & Zhang Z. 2024. Development and reproduction of four predatory mites (Parasitiformes: Phytoseiidae) feeding on the spider mites Tetranychus evansi and T. urticae (Trombidiformes: Tetranychidae) and the dried fruit mite Carpoglyphus lactis (Sarcoptiformes: Carpoglyphidae). Systematic and Applied Acarology 29(2): 269–284. https://doi.org/10.11158/saa.29.2.7
- Whittaker J. B. & Lewis T. 1975. Thrips: Their biology, ecology and economic importance. The Journal of Animal Ecology 44(1): 343. https://doi.org/10.2307/3870
- Womersley H. 1956. On some New Acarina-Mesostigmata from Australia, New Zealand and New Guinea. Journal of the Linnean Society of London, Zoology 42(288): 505–599. https://doi.org/10.1111/j.1096-3642.1956.tb02218.x
- Wu S., Gao Y., Xu X., Wang E., Wang Y. & Lei Z. 2014. Evaluation of Stratiolaelaos scimitus and Neoseiulus barkeri for biological control of thrips on greenhouse cucumbers. Biocontrol Science and Technology 24(10): 1110–1121. https://doi.org/10.1080/09583157.2014.924478
- Wu S., Reitz S. R., Qiu Z., He Z., Xing Z. & Gao Y. 2025. How have thrips succeeded as major pests globally? Entomologia Generalis 45(3): 609–619. https://doi.org/10.1127/entomologia/3084
- Xie L., Yan Y. & Zhang Z.-Q. 2018. Development, survival and reproduction of Stratiolaelaps scimitus (Acari: Laelapidae) on four diets. Systematic and Applied Acarology 23(4): 779–794. https://doi.org/10.11158/saa.23.4.16
- Xie L., Zhang K., Yan Y. & Zhang Z.-Q. 2025. Short-term influences of post-maturity diet switching on prey consumption and oviposition in the biocontrol agent Stratiolaelaps scimitus (Acari: Laelapidae). Systematic and Applied Acarology 30(6):1017–1027. https://doi.org/10.11158/saa.30.6.5
- Yang S.-H., Wang D., Chen C., Xu C.-L. & Xie H. 2020. Author correction: Evaluation of Stratiolaelaps scimitus (Acari: Laelapidae) for controlling the root-knot nematode, Meloidogyne incognita (Tylenchida: Heteroderidae). Scientific Reports 10(1): 9355. https://doi.org/10.1038/s41598-020-65968-0
- Yang Y., Zhang K. & Zhang Z.-Q. 2025. Predatory mites Amblydromalus limonicus and Amblyseius herbicolus (Acari: Phytoseiidae) as potential biocontrol agents of Eotetranychus sexmaculatus (Acari: Tetranychidae) in avocado: Examining predation on different prey life stages. Journal of Economic Entomology 118(3): 1335–1343. https://doi.org/10.1093/jee/toaf036
- Zhang K., Liu Z. & Zhang Z.-Q. 2024. Older mothers produce smaller eggs without compromising offspring quality: A study of a thelytokous mite predator (Acari: Phytoseiidae). Bulletin of Entomological Research 114(6): 820–827. https://doi.org/10.1017/S0007485324000658
- Zhang K. & Zhang Z.-Q. 2021. The dried fruit mite Carpoglyphus lactis (Acari: Carpoglyphidae) is a suitable alternative prey for Amblyseius herbicolus (Acari: Phytoseiidae). Systematic and Applied Acarology 26(11): 2167–2176. https://doi.org/10.11158/saa.26.11.15
- Zhang N., Zhang K., Liu Z., Yan Y., Zhang Z. & Xie L. 2025. Inbreeding depression in the predatory mite Stratiolaelaps scimitus: Demographic and transcriptomic insights. Pest Management Science ps.70274. https://doi.org/10.1002/ps.70274
- Zhang X., Lei Z., Reitz S. R., Wu S. & Gao Y. 2019. Laboratory and greenhouse evaluation of a granular formulation of Beauveria bassiana for control of western flower thrips, Frankliniella occidentalis. Insects 10(2): 58. https://doi.org/10.3390/insects10020058
- Zhang X., Wu S., Reitz S. R. & Gao Y. 2021. Simultaneous application of entomopathogenic Beauveria bassiana granules and predatory mites Stratiolaelaps scimitus for control of western flower thrips, Frankliniella occidentalis. Journal of Pest Science 94(1): 119–127. https://doi.org/10.1007/s10340-020-01227-5
- Zhang X., Zhang S., Zhu Z., Wang H., Yan Y. & Xie L. 2022. Review of predatory mites as biocontrol agents against thrips in China. Zoosymposia 22: 279–281. https://doi.org/10.11646/zoosymposia.22.1.176
- Zhou C.-Y., Zhang Q., Li Y.-Y., Yan Y. & Xie L.-X. 2018. Functional response of Stratiolaelaps scimitus to Bradysia odoriphaga. Yingyong Kunchongxuebao (Chinese Journal of Applied Entomology) 55(4): 705–710. https://doi.org/10.7679/j.issn.2095-1353.2018.088
- Zhu J., Cui X., Yang J., Lin X., Yan Y., Cai D. & Li J. 2023. Functional response of two soil-dwelling predatory mites, Macrocheles mammifer (Berlese) (Acari: Macrochelidae), and Stratiolaelaps scimitus (Womersley) (Acari: Laelapidae), on thrips Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae). Acarologia 63(4): 1039–1047. https://doi.org/10.24349/gbg7-7c27