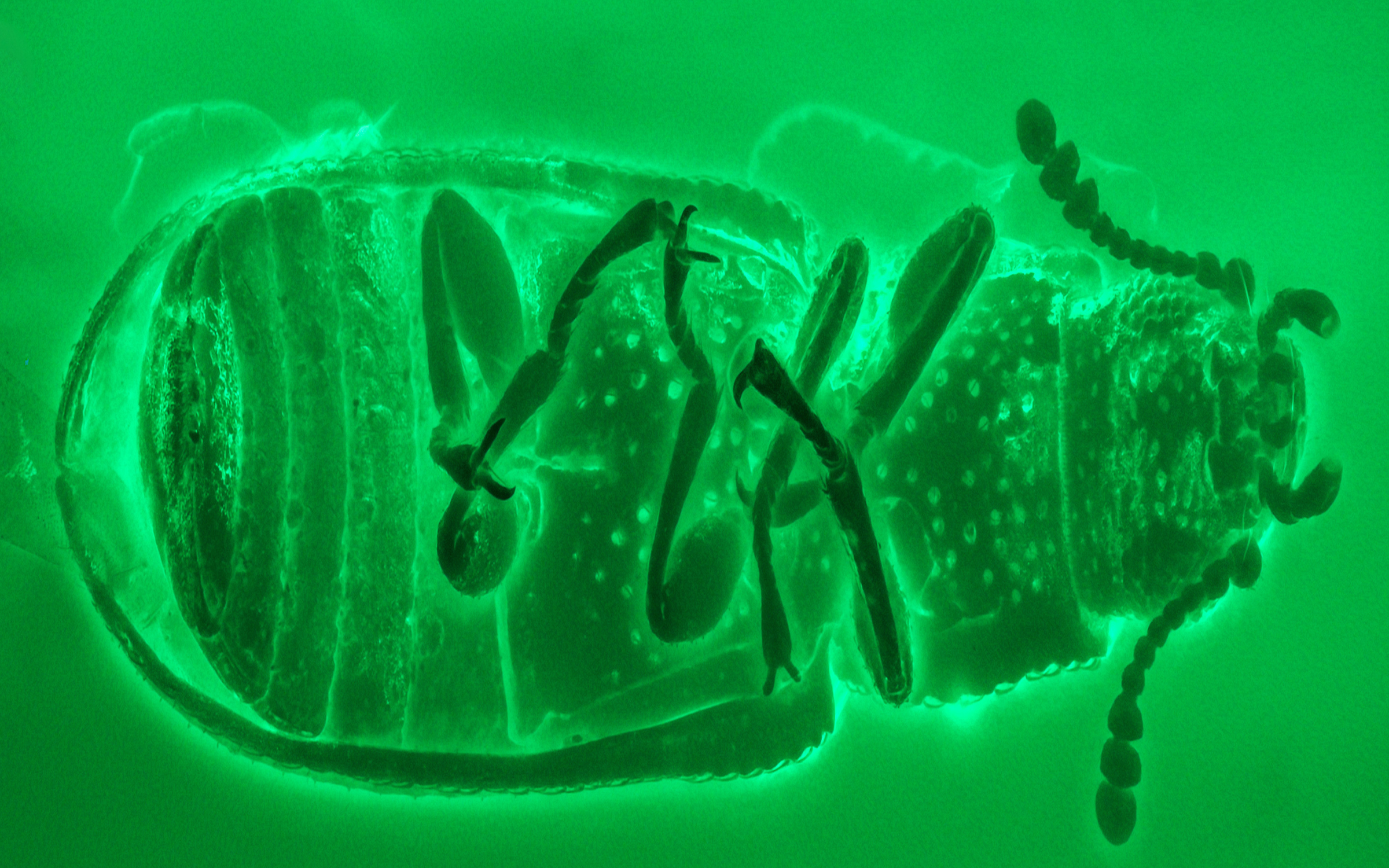
Abstract
Confocal laser scanning microscopy is an essential analytical tool in biological, biomedical, and material sciences, integrating microscope manufacturing technology, optical-electronic technology, and computer technology. In the last decade, confocal laser scanning microscopy has been successfully applied to the study of amber bioinclusions. Enhanced signal to noise ratios, resolution power, capability of optical sectioning, three-dimensional reconstruction, and better performance when imaging thicker samples provide a great deal of valuable and detailed morphological information about amber fossils. We briefly discuss the practical applications of CLSM in amber studies and compare it with other imaging methods commonly used in the field, including bright-field microscopy, wide-field fluorescence microscopy, and micro-computed tomography. A general procedure for imaging amber inclusions with CLSM is provided, with a focus on pretreatments and image processing.
References
- Ascaso, C., Wierzchos, J., Corral, J.C., Lopez, C. & Alonso, J. (2003) New application of light and electron microscopic techniques for the study of microbial inclusions in amber. Journal of Paleontology, 77, 986–996. https://doi.org/10.1666/0022-3360(2003)077<1182:NAOLAE>2.0.CO;2
- Ascaso, C., Wierzchos, J., Speranza, M., Gutiérrez, J.C., González, A.M., de los Ríos, A. & Alonso, J. (2005) Fossil protists and fungi in amber and rock substrates. Micropaleontology, 51, 59–72. https://doi.org/10.2113/51.1.59
- Arillo, A., Subías, L.S. & Sánchez-García, A. (2016) New species of fossil oribatid mites (Acariformes, Oribatida), from the Lower Cretaceous amber of Spain. Cretaceous Research, 63, 68–76. https://doi.org/10.1016/j.cretres.2016.02.009
- Azar, D., Perrichot, V., Neraudeau, D. & Nel, A. (2003) New psychodid flies from the Cretaceous ambers of Lebanon and France, with a discussion about Eophlebotomus connectens Cockerell, 1920 (Diptera, Psychodidae). Annals of the Entomological Society of America, 96, 117–127. https://doi.org/10.1603/0013-8746(2003)096[0117:NPFTCA]2.0.CO;2
- Bao, T., Wang, B., Li, J.G. & Dilcher, D. (2019) Pollination of Cretaceous flowers. Proceedings of the National Academy of Sciences, USA, 116, 24707–24711. https://doi.org/10.1073/pnas.1916186116
- Böker, C. & Brocksch, D. (2002) Fascinating amber. Carl Zeiss: Innovation, 12, 20–23.
- Borlinghaus, R.T. (2017) The white confocal: microscopic optical sectioning in all colours. Springer International Publishing, Cham, 115 pp. https://doi.org/10.1007/978-3-319-55562-1
- Bonato, L., Edgecombe, G.D. & Minelli, A. (2014) Geophilomorph centipedes from the Cretaceous amber of Burma. Palaeontology, 57, 97–110. https://doi.org/10.1111/pala.12051
- Cai, C.Y. & Huang, D.Y. (2014) The oldest micropepline beetle from Cretaceous Burmese amber and its phylogenetic implications (Coleoptera: Staphylinidae). Naturwissenschaften, 101, 813–817. https://doi.org/10.1007/s00114-014-1221-z
- Cai, C.Y., Clarke, D.J., Yin, Z.W, Fu, Y.Z. & Huang, D.Y. (2019) A specialized prey-capture apparatus in mid-Cretaceous rove beetles. Current Biology, 29, R105–R119. https://doi.org/10.1016/j.cub.2019.01.002
- Cai, C.Y., Thayer, M.K., Newton, A.F., Yin, Z.W. & Huang, D.Y. (2018) A new genus of dasycerine rove beetles from Upper Cretaceous Burmese amber and its phylogenetic implications (Coleoptera, Staphylinidae). Cretaceous Research, 84, 431–436. https://doi.org/10.1016/j.cretres.2017.12.004
- Chen, D.J., Yang, J. & Shao, Y.W. (2019) Amber insects. Chemical Industry Press, Beijing, pp. 1–274 [in Chinese].
- Christiansen, K. & Nascimbene, P. (2006) Collembola (Arthropoda, Hexapoda) from the mid Cretaceous of Myanmar (Burma). Cretaceous Research, 27, 318–363. https://doi.org/10.1016/j.cretres.2005.07.003
- Claxton, N.S., Fellers, T.J. & Davidson, M.W. (2006) Laser scanning confocal microscopy. In: Webster, J.G. (Ed.), Encyclopedia of medical devices and instrumentation. New Jersey, John Wiley & Sons. pp. 449–477. https://doi.org/10.1002/0471732877.emd291
- Clark, N.D.L. & Daly, C. (2010) Using confocal laser scanning microscopy to image trichome inclusions in amber. Journal of Paleontological Techniques, 8, 1–7.
- Cox, G. & Sheppard, C.J. (2004) Practical limits of resolution in confocal and non-linear microscopy. Microscopy Research and Technique, 63, 18–22. https://doi.org/10.1002/jemt.10423
- Cruickshank, R.D. & Ko, K. (2003) Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences, 21, 441–455. https://doi.org/10.1016/S1367-9120(02)00044-5
- Fu, Y.Z., Azar, D. & Huang, D.Y. (2020) A new species of the extinct family Minlagerrontidae (Insecta: Hemiptera: Cicadomorpha) from mid-Cretaceous Burmese amber. Cretaceous Research, 107, 104270. https://doi.org/10.1016/j.cretres.2019.104270
- Fu, Y.Z., Szwedo, J. & Huang, D.Y. (2021) A new sinoalid froghopper in mid-Cretaceous amber from northern Myanmar (Hemiptera, Cicadomorpha, Sinoalidae). Cretaceous Research, 125, 104841. https://doi.org/10.1016/j.cretres.2021.104841
- González, S. & Halpern, A. (2007) Laser-scanning confocal microscopy. In: Soyer, H.P., Argenziano, G., Hofmann-Wellenhof , R. & Johr, R.H. (Eds), Colour atlas of melanocytic lesions of the skin. Springer, Berlin, Heidelberg, pp. 39–46. https://doi.org/10.1007/978-3-540-35106-1_5
- Compton, S.G., Ball, A.D., Collinson, M.E., Hayes, P., Rasnitsyn, A.P. & Ross, A.J. (2010) Ancient fig wasps indicate at least 37 Myr of stasis in their mutualism with fig trees. Biology Letters, 6, 838–842. https://doi.org/10.1098/rsbl.2010.0389
- Guo, M.X., Yang, H.D., Li, G., Tong, Y.-J., Li, S., Lu, Y.Y., Shi, A.M., Wang, B., Zhang, W.W. & Bai, M. (2016) Morphological identifiability of Burmese amber inclusions under X-rays. Acta Entomologica Sinica, 59, 1013–1020 [In Chinese]. https://doi.org/10.16380/j.kcxb.2016.09.012
- Halbhuber, K.J. & Konig, K. (2003) Modern laser scanning microscopy in biology, biotechnology and medicine. Annals of Anatomy, 185, 1–20. https://doi.org/10.1016/S0940-9602(03)80002-X
- Heethoff, M., Helfen, L. & Norton, R.A. (2009) Description of Neoliodes dominicus n. sp. (Acari, Oribatida) from Dominican amber, aided by synchrotron X-ray microtomography. Journal of Paleontology, 83, 153–159. https://doi.org/10.1666/08-101R1.1
- Hein, H.J., Czurratis, P., Schroth, D. & Bernstein, A. (1995) A comparative study of the application of scanning acoustic microscopy and confocal laser scanning microscopy to the structural assessment of human bones. Annals of Anatomy, 177, 427–430. https://doi.org/10.1016/S0940-9602(11)80149-4
- Hovis, D.B. & Heuer, A.H. (2010) The use of laser scanning confocal microscopy (LSCM) in materials science. Journal of Microscopy, 240, 173–180. https://doi.org/10.1111/j.1365-2818.2010.03399.x
- Kirejtshuk, A.G., Chetverikov, P.E. & Azar, D. (2015) Libanopsinae, new subfamily of the family Sphindidae (Coleoptera, Cucujoidea) from Lower Cretaceous Lebanese amber, with remarks on using confocal microscopy for the study of amber inclusions. Cretaceous Research, 52, 461–479. https://doi.org/10.1016/j.cretres.2014.02.008
- Kundrata, R., Bukejs, A., Prosvirov, A.S. & Hoffmannova, J. (2020) X-ray micro-computed tomography reveals a unique morphology in a new click-beetle (Coleoptera, Elateridae) from the Eocene Baltic amber. Scientific Reports, 10, 20158. https://doi.org/10.1038/s41598-020-76908-3
- Kypke, J.L. & Solodovnikov, A. (2020) Every cloud has a silver lining: X-ray micro-CT reveals Orsunius rove beetle in Rovno amber from a specimen inaccessible to light microscopy. Historical Biology, 32, 940–950. https://doi.org/10.1080/08912963.2018.1558222
- Lak, M., Néraudeau, D., Nel, A., Cloetens, P., Perrichot, V. & Tafforeau, P. (2008) Phase contrast X-ray synchrotron imaging: opening access to fossil inclusions in opaque amber. Microscopy and Microanalysis, 14, 251–259. https://doi.org/10.1017/S1431927608080264
- Li, Y.D., Yamamoto, S., Huang, D.Y. & Cai, C.Y. (2020a) Confocal and micro-CT data of Miniomma chenkuni, holotype, NIGP173375. Zenodo. https://doi.org/10.5281/zenodo.3994920
- Li, Y.D., Yamamoto, S., Huang, D.Y. & Cai, C.Y. (2020b) A miniaturized ommatid beetle in mid-Cretaceous Burmese amber (Coleoptera: Archostemata: Ommatidae). Papéis Avulsos de Zoologia, 60, e20206063. https://doi.org/10.11606/1807-0205/2020.60.63
- Li, Y.D., Tihelka, E., Leschen, R.A.B., Yu, Y., Ślipiński, A., Pang, H., Huang, D.Y., Kolibáč, J. & Cai, C.Y. (2021a) Confocal and micro-CT data of Microtrogossita qizhihaoi, holotype, NIGP173910. Zenodo.
- Li, Y.D., Yamamoto, S., Huang, D.Y. & Cai, C.Y. (2021b) Confocal and micro-CT data of Paraodontomma leptocristatum, holotype, NIGP174676. Zenodo. https://doi.org/10.5281/zenodo.4737033
- Li, Y.D., Yamamoto, S., Huang, D.Y. & Cai, C.Y. (2021c) New species of Paraodontomma from mid-Cretaceous Burmese amber with muscle tissue preservation (Coleoptera: Archostemata: Ommatidae). Papéis Avulsos de Zoologia, 61, e20216153. https://doi.org/10.11606/1807-0205/2021.61.53
- Li, Y.D., Huang, D.Y. & Cai, C.Y. (2021d) Confocal data of Pseudomataeopsephus burmensis, holotype, NIGP173913. Zenodo.
- Liu, Y.M., Hakim, M. & Huang, D.Y. (2020) First stratiomyomorphan larvae in the mid-Cretaceous amber from Myanmar (Diptera: Brachycera). Cretaceous Research, 106, 104256. https://doi.org/10.1016/j.cretres.2019.104265
- Martišek, D. (2017) 3D reconstruction of the surface using a standard camera. Mathematical Problems in Engineering, 2017, 4642397. https://doi.org/10.1155/2017/4642397
- Paddock, S.W. (2000) Principles and practices of laser scanning confocal microscopy. Molecular Biotechnology, 16, 127–149. https://doi.org/10.1385/MB:16:2:127
- Perreau, M. & Tafforeau, P. (2011) Virtual dissection using phase-contrast X-ray synchrotron microtomography: reducing the gap between fossils and extant species. Systematic Entomology, 36, 573–580. https://doi.org/10.1111/j.1365-3113.2011.00573.x
- Peyrot, D., Barrón, E., Pereda-Suberbiola, X. & Company, J. (2020) Vegetational composition of the Upper Cretaceous vertebrate site of Chera (Valencia, Spain) and its significance in mosaic vegetation from southwestern Europe. Cretaceous Research, 106, 104254. https://doi.org/10.1016/j.cretres.2019.104254
- Prasad, V., Farooqui, A., Murthy, S., Sarate, O.S. & Bajpai, S. (2018) Palynological assemblage from the Deccan Volcanic Province, central India: insights into early history of angiosperms and the terminal Cretaceous paleogeography of peninsular India. Cretaceous Research, 86, 186–198. https://doi.org/10.1016/j.cretres.2018.03.004
- Robin, N., Béthoux, O., Sidorchuk, E., Cui, Y., Li, Y., Germain, D., King, A., Berenguer, F. & Ren, D. (2016) A Carboniferous mite on an insect reveals the antiquity of an inconspicuous interaction. Current Biology, 26, 1376–1382. https://doi.org/10.1016/j.cub.2016.03.068
- Ross, A.J. (2019) Burmese (Myanmar) amber checklist and bibliography 2018. Palaeoentomology, 2 (1), 22–84. https://doi.org/10.11646/palaeoentomology.2.1.5
- Ross, A.J. (2021) Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2020. Palaeoentomology, 4 (1), 57–76. https://doi.org/10.11646/palaeoentomology.4.1.11
- Shihavuddin, A., Basu, S., Rexhepaj, E., Delestro, F., Menezes, N., Sigoillot, S.M., Nery, E.D., Selimi, F, Spassky, N. & Genovesio, A. (2017) Smooth 2D manifold extraction from 3D image stack. Nature Communications, 8, 15554. https://doi.org/10.1038/ncomms15554
- Sidorchuk, E.A. (2013) New technique for preparation of small-sized amber samples with application to mites. In: Azar, D., Engel, M.S., Jarzembowski, E., Krogmann, L., Nel, A. & Santiago-Blay, J.A. (Eds., .Insect Evolution in an amberiferous and stone alphabet. Proceedings of the 6th International Congress on Fossil Insects, Arthropods and Amber. Brill, Leiden-Boston. pp. 189–201. https://doi.org/10.1163/9789004210714_014
- Sidorchuk, E.A. & Vorontsov, D.D (2018) Preparation of small-sized 3D amber samples: state of the technique. Palaeoentomology, 1 (1), 80–90. https://doi.org/10.11646/palaeoentomology.1.1.10
- Speranza, M., Wierzchos, J., Alonso, J., Bettucci, L., Martín-González, A. & Ascaso, C. (2010) Traditional and new microscopy techniques applied to the study of microscopic fungi included in amber. In: Méndez-Vilas, A. & Díaz, J. (Eds) Microscopy: science, technology, applications and education. Badajoz, Spain, pp. 1135–1145.
- Su, Y.T., Cai, C.Y. & Huang, D.Y. (2019) Revision of Phryssonotus burmiticus (Diplopoda, Polyxenida, Synxenidae) in mid-Cretaceous amber from Myanmar. Cretaceous Research, 93, 216–224. https://doi.org/10.1016/j.cretres.2018.09.002
- Su, Y.T., Cai, C.Y. & Huang, D.Y. (2020) Two new species of the bristle millipede genus Pauropsxenus (diplopoda, Polyxenidae) in mid-Cretaceous Burmese amber. Cretaceous Research, 111, 104427. https://doi.org/10.1016/j.cretres.2020.104427
- Tihelka, E., Huang, D.Y. & Cai, C.C. (2020) New data on Ommatidae (Coleoptera) from mid-Cretaceous Burmese amber. Cretaceous Research, 106, 104253. https://doi.org/10.1016/j.cretres.2019.104253
- Tihelka, E., Li, L.Q., Fu, Y.Z., Su, Y.T., Huang, D.Y. & Cai, C.Y. (2021) Angiosperm pollinivory in a Cretaceous beetle. Nature Plants, 7, 445–451. https://doi.org/10.1038/s41477-021-00893-2
- Van de Kamp, T., Dos Santos Rolo, T., Baumbach, T. & Krogmann, L. (2014) Scanning the past-synchrotron X-ray microtomography of fossil wasps in amber. Entomologie heute, 26, 151–160.
- Xiao, Y.M., Fu, D.L. & Li, A.S. (1999) Laser scanning confocal microscope (LSCM) and its application in biology. Acta Laser Biology Sinica, 8, 305–311 [in Chinese]. https://doi.org/10.1007/BF02946523
- Yin, Z.W., Chandler, D.S. & Cai, C.Y. (2019) Priscaplectus gen. nov. and two new species in mid-Cretaceous amber from Myanmar (Coleoptera: Staphylinidae: Pselaphinae). Cretaceous Research, 103, 104174. https://doi.org/10.1016/j.cretres.2019.07.004
- Yin, Z.W., Lü, L., Yamamoto, S, Thayer, M.K., Newton, A.F. & Cai, C.Y. (2021) Dasycerinae rove beetles: Cretaceous diversification, phylogeny and historical biogeography (Coleoptera: Staphylinidae: Dasycerinae). Cladistics, 37, 185–210. https://doi.org/10.1111/cla.12430
- Zhang, Q.Q., Zhang, J.F. & Wang, B. (2017) First record of the subfamily Archinemestriinae in the family Nemestrinidae (Diptera: Brachycera) from Upper Cretaceous Burmese amber. Cretaceous Research, 75, 141–145. https://doi.org/10.1016/j.cretres.2017.03.005
- Zhao, X.D, Zhang, Q.Q., Jarzembowski, E.A., Chen, L. & Wang, B. (2016) A new earwingfly from mid-Cretaceous Burmese amber (Mecoptera: Meropeidae). Cretaceous Research, 66, 136–140. https://doi.org/10.1016/j.cretres.2016.06.008