Abstract
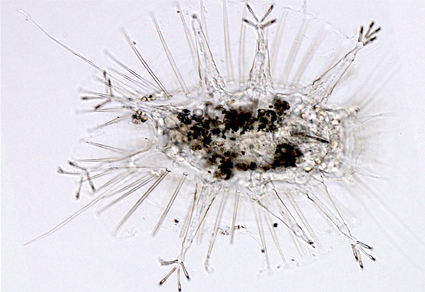
Little is still known about the diversity and evolution of marine arthrotardigrades, as they are generally difficult to sample, resulting in a limited amount of molecular data for barcoding and phylogenetic studies. With the current study, we provide the first investigation into COI haplotype diversity in a marine tanarctid and at the same time readdress arthrotardigrade phylogeny. Specifically, we provide COI mtDNA, 18S and 28S rDNA sequences from a population of Actinarctus doryphorus (Tanarctidae) sampled off the coast of Roscoff, France and further provide new 18S sequences from two marine echiniscoidids. A. doryphorus COI sequences confirmed the presence of a single species and further revealed five haplotypes shared among nine sequenced individuals. Our 18S and 28S rDNA datasets were individually and combined analysed with Bayesian inference and Maximum Likelihood. Actinarctus doryphorus was placed together with Tanarctus sequences within a maximally supported Tanarctidae, confirming previous interpretations that the clade is distinct from Halechiniscidae. Although several studies in recent decades have concluded that the marine arthrotardigrades are paraphyletic, recent studies have argued that the clade may not be paraphyletic. Our phylogenetic analyses consistently inferred Arthrotardigrada as paraphyletic, as the clade includes the monophyletic Echiniscoidea. Accordingly, we propose that it is time to suppress the order Arthrotardigrada as it clearly does not reflect tardigrade phylogeny.
References
- Bertolani, R., Guidetti, R., Marchioro, T., Altiero, T., Rebecchi, L. & Cesari, M. (2014) Phylogeny of Eutardigrada: new molecular data and their morphological support lead to the identification of new evolutionary lineages. Molecular Phylogenetics and Evolution, 76, 110–126. https://doi.org/10.1016/j.ympev.2014.03.006
- Blaxter, M.L., De Ley, P., Garey, J.R., Liu, L.X., Scheldeman, P., Vierstraete, A., Vanfleteren, J.R., Mackey, L.Y., Dorris, M., Frisse, L.M., Vida, J.T. & Thomas, W.K. (1998) A molecular evolutionary framework for the phylum Nematoda. Nature, 392 (6671), 71–75. https://doi.org/10.1038/32160
- Cádiz, A., Nagata, N., Díaz, L.M., Suzuki-Ohno, Y., Echenique-Díaz, L.M., Akashi, H.D., Makino, T. & Kawata, M. (2018) Factors affecting interspecific differences in genetic divergence among populations of Anolis lizards in Cuba. Zoological Letters, 4 (1), 21. https://doi.org/10.1186/s40851-018-0107-x
- Capella-Gutiérrez, S., Silla-Martínez, J.M. & Gabaldón, T. (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics, 25 (15), 1972–1973. https://doi.org/10.1093/bioinformatics/btp348
- Chapman, P.A., Owen, H., Flint, M., Traub, R.J., Cribb, T.H. & Mills, P.C. (2016) Molecular characterization of coccidia associated with an epizootic in green sea turtles (Chelonia mydas) in South East Queensland, Australia. PLoS One, 11 (2), e0149962. https://doi.org/10.1371/journal.pone.0149962
- Chernomor, O., Von Haeseler, A. & Minh, B.Q. (2016) Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology, 65 (6), 997–1008. https://doi.org/10.1093/sysbio/syw037
- Cuénot, L. (1892) Tardigrades. Commensaux et parasites des Echinodermes, I. Revue Biologique du Nord de la France, 5, 1–22.
- Degma, P. & Guidetti, R. (2009–2023) Actual checklist of Tardigrada species. 2009–2023, 42th Edition: 09-01-2023, 67 pp. Available from: https://iris.unimore.it/handle/11380/1178608 (accessed 14 February 2023) https://doi.org/10.25431/11380_1178608
- De la Torre-Bárcena, J.E., Kolokotronis, S-O., Lee, E.K., Stevenson, D.W., Brenner, E.D., Katari, M.S., Coruzzi, G.M. & DeSalle, R. (2009) The impact of outgroup choice and missing data on major seed plant phylogenetics using genome-wide EST data. PLoS One, 4 (6), e5764. https://doi.org/10.1371/journal.pone.0005764
- Edgar, R.C. (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics, 5 (1), 113. https://doi.org/10.1186/1471-2105-5-113
- Fleming, J.F. & Arakawa, K. (2021) Systematics of Tardigrada: a reanalysis of tardigrade taxonomy with specific reference to Guil et al. (2019). Zoologica Scripta, 50 (3), 376–382. https://doi.org/10.1111/zsc.12476
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3 (5), 294–299.
- Fujimoto, S., Miyazaki, K. & Suzuki, A.C. (2013) A new marine tardigrade, Tanarctus diplocerus (Arthrotardigrada: Halechiniscidae) from Japan. Journal of the Marine Biological Association of the United Kingdom, 93 (4), 955–961. https://doi.org/10.1017/S0025315412000689
- Fujimoto, S. (2014) A new Stygarctus (Arthrotardigrada: Stygarctidae) from Japan, with entangled seminal receptacle ducts. Zootaxa, 3784 (2), 187–195. https://doi.org/10.11646/zootaxa.3784.2.8
- Fujimoto, S. (2015) Halechiniscidae (Heterotardigrada, Arthrotardigrada) of Oura Bay, Okinawajima, Ryukyu Islands, with descriptions of three new species. ZooKeys 483: 149–166. https://doi.org/10.3897/zookeys.483.8936
- Fujimoto, S, Jørgensen, A. & Hansen, J.G. (2017) A molecular approach to arthrotardigrade phylogeny (Heterotardigrada, Tardigrada). Zoologica Scripta, 46, 496–505. https://doi.org/10.1111/zsc.12221
- Fujimoto, S., Suzuki, A.C., Ito, M., Tamura, T. & Tsujimoto, M. (2020) Marine tardigrades from Lützow-Holm Bay, East Antarctica with the description of a new species. Polar Biology, 43, 679–693. https://doi.org/10.1007/s00300-020-02671-w
- Gąsiorek, P. & Michalczyk, Ł. (2020) Phylogeny of Itaquasconinae in the light of the evolution of the flexible pharyngeal tube in Tardigrada. Zoologica Scripta, 49 (4), 499–515. https://doi.org/10.1111/zsc.12424
- Gąsiorek, P. & Kristensen, R.M. (2022) New marine heterotardigrade lineages (Echiniscoididae) from the tropics. The European Zoological Journal, 89 (1), 719–754. https://doi.org/10.1080/24750263.2022.2079737
- Gross, V., Epple, L. & Mayer, G. (2021) Organization of the central nervous system and innervation of cephalic sensory structures in the water bear Echiniscus testudo (Tardigrada: Heterotardigrada) revisited. Journal of Morphology, 282 (9), 1298–1312. https://doi.org/10.1002/jmor.21386
- Giribet, G., Carranza, S., Baguna, J., Riutort. M. & Ribera, C. (1996) First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Molecular Biology and Evolution, 13 (1), 76–84. https://doi.org/10.1093/oxfordjournals.molbev.a025573
- Giribet, G. & Edgecombe, G.D. (2017) Current understanding of Ecdysozoa and its internal phylogenetic relationships. Integrative and Comparative Biology, 57 (3), 455–466. https://doi.org/10.1093/icb/icx072
- Guil, N. & Giribet, G. (2009) Fine scale population structure in the Echiniscus blumi-canadensis series (Heterotardigrada, Tardigrada) in an Iberian mountain range—When morphology fails to explain genetic structure. Molecular Phylogenetics and Evolution, 51 (3), 606–13. https://doi.org/10.1016/j.ympev.2009.02.019
- Guil, N., Jørgensen, A., Giribet, G. & Kristensen, R.M. (2013) Congruence between molecular phylogeny and cuticular design in Echiniscoidea (Tardigrada, Heterotardigrada). Zoological Journal of the Linnean Society, 169 (4), 713–736. https://doi.org/10.1111/zoj.12090
- Guil, N., Jørgensen, A. & Kristensen, R. (2019) An upgraded comprehensive multilocus phylogeny of the Tardigrada tree of life. Zoologica Scripta, 48 (1), 120–137. https://doi.org/10.1111/zsc.12321
- Hansen, J., Kristensen, R.M. & Jørgensen, A. (2012) The armoured marine tardigrades (Arthrotardigrada, Tardigrada). Vol. 2. The Royal Danish Academy of Sciences and Letters, Copenhagen, 91 pp.
- Huelsenbeck, J.P. & Rannala, B. (2004) Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Systematic Biology, 53 (9), 904–913. https://doi.org/10.1080/10635150490522629
- Huelsenbeck, J.P. & Ronquist, F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17 (8), 754–755. https://doi.org/10.1093/bioinformatics/17.8.754
- Jørgensen, A., Møbjerg, N. & Kristensen, R.M. (2007) A molecular study of the tardigrade Echiniscus testudo (Echiniscidae) reveals low DNA sequence diversity over a large geographical area. Journal of Limnology, 66, 77–83. https://doi.org/10.4081/jlimnol.2007.s1.77
- Jørgensen, A., Faurby, S., Hansen, J.G., Møbjerg, N. & Kristensen, R.M. (2010) Molecular phylogeny of Arthrotardigrada (Tardigrada). Molecular Phylogenetics and Evolution, 54 (3), 1006–1015. https://doi.org/10.1016/j.ympev.2009.10.006
- Jørgensen, A., Faurby, S., Persson, D.K., Halberg, A.K., Kristensen, R. & Møbjerg, N. (2013) Genetic diversity in the parthenogenetic reproducing tardigrade Echiniscus testudo (Heterotardigrada: Echiniscoidea). Journal of Limnology, 72 (s1), e17. https://doi.org/10.4081/jlimnol.2013.s1.e17
- Jørgensen, A., Boesgaard, T.M., Møbjerg, N. & Kristensen, R.M. (2014) The tardigrade fauna of Australian marine caves: With descriptions of nine new species of Arthrotardigrada. Zootaxa, 3802 (4), 401–443. https://doi.org/10.11646/zootaxa.3802.4.1
- Jørgensen, A., Kristensen, R.M. & Møbjerg, N. (2018) Phylogeny and integrative taxonomy of Tardigrada. In: Schill, R.O. (Ed.), Water Bears: The Biology of Tardigrades. Vol. 2. Springer, Cham, pp. 95–114. https://doi.org/10.1007/978-3-319-95702-9_3
- Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., Von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14 (6), 587–589. https://doi.org/10.1038/nmeth.4285
- Kristensen, R.M. (1981) Sense organs of two marine arthrotardigrades (Heterotardigrada, Tardigrada). Acta Zoologica, 62 (1), 27–41. https://doi.org/10.1111/j.1463-6395.1981.tb00614.x
- Kumar, S., Stecher, G. & Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33 (7), 1870–1874. https://doi.org/10.1093/molbev/msw054
- Madeira, F., Park, Y.M., Lee, J., Buso, N., Gur, T., Madhusoodanan, N., Basutkar, P., Tivey, A.R.N., Potter, S.C., Finn, R.D. & Lopez, R. (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Research, 47 (W1), W636–W641. https://doi.org/10.1093/nar/gkz268
- Møbjerg, N., Kristensen, R.M. & Jørgensen, A. (2016) Data from new taxa infer Isoechiniscoides gen. nov. and increase the phylogenetic and evolutionary understanding of echiniscoidid tardigrades (Echiniscoidea: Tardigrada). Zoological Journal of the Linnean Society, 178 (4), 804–818. https://doi.org/10.1111/zoj.12500
- Møbjerg, N., Jørgensen, A., Kristensen, R.M. & Neves, R.C. (2018) Morphology and functional anatomy. In: Schill, R.O. (Ed.), Water Bears: The Biology of Tardigrades. Vol. 2. Springer, Switzerland, pp. 57– 95. https://doi.org/10.1007/978-3-319-95702-9_2
- Møbjerg, N., Jørgensen, A. & Kristensen, R.M. (2020) Ongoing revision of Echiniscoididae (Heterotardigrada: Echiniscoidea), with the description of a new interstitial species and genus with unique anal structures. Zoological Journal of the Linnean Society, 188 (3), 663–680. https://doi.org/10.1093/zoolinnean/zlz122
- Morek, W., Surmacz, B., López-López, A. & Michalczyk, Ł. (2021) “Everything is not everywhere”: time-calibrated phylogeography of the genus Milnesium (Tardigrada). Molecular Ecology, 30 (14), 3590–3609. https://doi.org/10.1111/mec.15951
- Neves, R.C, Kristensen R.M. & Møbjerg, N. 2021.New records on the rich loriciferan fauna of Trezen ar Skoden (Roscoff, France): Description of two new species of Nanaloricus and the new genus Scutiloricus. PLoS ONE, 16 (5), e0250403. https://doi.org/10.1371/journal.pone.0250403
- Nguyen. L.T., Schmidt, H.A., Von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32 (1), 268–274. https://doi.org/10.1093/molbev/msu300
- Persson, D.K., Halberg, K.A., Jørgensen, A., Møbjerg, N. & Kristensen, R.M. (2012) Neuroanatomy of Halobiotus crispae (Eutardigrada: Hypsibiidae): Tardigrade brain structure supports the clade Panarthropoda. Journal of Morphology, 273 (11), 1227–1245. https://doi.org/10.1002/jmor.20054
- Persson, D.K., Halberg, K.A., Neves, R.C., Jørgensen, A., Kristensen, R.M. & Møbjerg, N. (2019) Comparative myoanatomy of Tardigrada: new insights from the heterotardigrades Actinarctus doryphorus (Tanarctidae) and Echiniscoides sigismundi (Echiniscoididae). BMC Evolutionary Biology, 19 (1), 206. https://doi.org/10.1186/s12862-019-1527-8
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarization in bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67 (5), 901–904. https://doi.org/10.1093/sysbio/syy032
- Renaud-Mornant, J. (1971) Campagne d`essais du “Jean Charcot” (3 - 8 Décembre 1968) 8. Méiobenthos. II. Tardigrades. Bulletin du Muséum National d’Histoire Naturelle, Paris, Série 2, 42, 957–969.
- Renaud-Mornant, J. (1981) Raiarctus colurus n.g., n. sp., et R. aureolatus n. sp., tardigrades (Arthrotardigrada) marins nouveaux de sédiments calcaires. Bulletin du Muséum National d’Histoire Naturelle, 3 (2), 512–522.
- Renaud-Mornant, J. (1982) Species diversity in marine Tardigrada. In: Nelson, D.R. (Ed.), Proceedings of the Third International Symposium on Tardigrada, 1980. Tennessee State University Press, Nashville, Tennessee, pp. 149–178.
- Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19 (12), 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
- Rozas, J., Ferrer-Mata, A., Sánchez-DelBarrio, J.C., Guirao-Rico, S., Librado, P., Ramos-Onsins, S.E. & Sánchez-Gracia, A. (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution, 34 (12), 3299–3302. https://doi.org/10.1093/molbev/msx248
- Ryu, S.H., Lee, J.M., Jang, K.H., Choi, E.H., Park, S.J., Chang, C.Y., Kim, W. & Hwang, U.W. (2007) Partial mitochondrial gene arrangements support a close relationship between Tardigrada and Arthropoda. Molecules & Cells, 24 (3), 351–357.
- Schulz, E. (1955) Studien an marinen Tardigraden. Kieler Meeresforschungen, 11 (1), 74–79.
- Schwendinger, P.J. & Giribet, G. (2005) The systematics of the south-east Asian genus Fangensis Rambla (Opiliones: Cyphophthalmi: Stylocellidae). Invertebrate Systematics, 19 (4), 297–323. https://doi.org/10.1071/IS05023
- Stec, D., Krzywański, Ł., Arakawa, K. & Michalczyk, Ł. (2020) A new redescription of Richtersius coronifer, supported by transcriptome, provides resources for describing concealed species diversity within the monotypic genus Richtersius (Eutardigrada). Zoological Letters, 6, 1–25. https://doi.org/10.1186/s40851-020-0154-y
- Whiting, M.F. (2002) Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zoologica Scripta, 31 (1), 93–104. https://doi.org/10.1046/j.0300-3256.2001.00095.x
- Whiting, M.F., Carpenter, J.C., Wheeler, Q.D. & Wheeler, W.C. (1997) The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology, 46 (1), 1–68. https://doi.org/10.1093/sysbio/46.1.1