Abstract
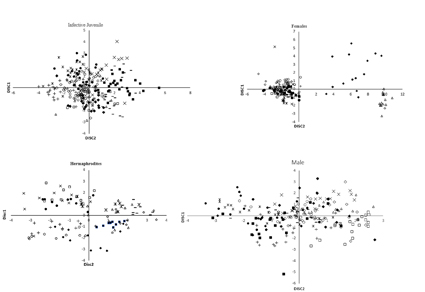
In a survey of entomopathogenic nematodes (EPNs) in the northwest of Iran, eleven isolates of Heterorhabditis bacteriophora were recovered mainly from the soil samples of apple orchards. The isolates were identified morphologically and molecularly. To unravel intraspecific variation, morphometric characters of different life stages, including hermaphrodite, female, male and infective juvenile (IJ), were evaluated to see efficiencies in discrimination of H. bacteriophora populations isolated from relatively limited geographical regions. Morphometric data were subjected to principal component analysis (PCA), canonical analysis (CAN), and also cluster analysis. Significant differences in morphometrics were recorded between the studied populations. The results of the PCA on IJ variables showed that the first two PC account for more than 99% of the total variance. Variables including distance from anterior end to the base of the pharynx (Es) and distance from anterior end to nerve ring (Nr) showed the highest factor loading. Cluster analysis put the studied populations in five well-defined groups. Resulted PC1 and PC2 for females account 63% of the total variance. Females variables include body length (L), distance from anterior end to excretory pore (EP), length divided by width (A), length divided by Es (B), length divided by tail length (C), and anal body width (Abw), tail length (TL), EP divided by TL ×100 (E) were effective in discrimination of the populations into three distinct groups. The first two PC from hermaphrodite variables accounted for 66% of the total variance. Variables include L, A, B, and C had efficient factor loading on PC1, and PC2. Cluster analysis of hermaphrodite morphometrics yielded five distinct groups among populations. For male variables PC1, and PC2 accounted 60.04% of the total variance, and body width, Abw, TL and, E were the most efficient in PC1 and PC2 was affected efficiently by Es and EP. The results of PCA showed that the highest discrimination among the studied H. bacteriophora population occurred with female variables.
References
- Abdolmaleki, A., Tanha Maafi, Z., Dastjerdi, H.R., Naseri, B. & Ghasemi, A. (2016) Isolation and identification of entomopathogenic nematodes and their symbiotic bacteria from Kurdistan province in Iran. Journal of Crop Protection, 5 (2), 259–271. https://doi.org/10.18869/modares.jcp.5.2.259
- Achinelly, M.F., Eliceche, D.P., Belaich, M.N. & Ghiringhelli, P.D. (2017) Variability study of entomopathogenic nematode populations (Heterorhabditidae) from Argentina. Brazilian Journal of Biology, 77 (3), 569–579. https://doi.org/10.1590/1519-6984.20015
- Adams, B.J., Burnell, A.M. & Powers, T.O. (1998) A phylogenetic analysis of heterorhabditis (nemata: rhabditidae) based on internal transcribed spacer 1 DNA sequence data. Journal of Nematology, 30 (1), 22–39. [http://www.ncbi.nlm.nih.gov/pubmed/19274196]
- Agazadeh, M., Mohammadi, D. & Eivazian Kary, N. (2010) Distribution of Entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in Potato fields in North-west Iran. Munis Entomology and Zoology, 5 (2), 758–763.
- Bedding, R.A. & Akhurst, R.J. (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica, 21, 109–110. https://doi.org/10.1163/187529275X00419
- Boemare, N.E., Akhurst, R.J. & Mourant, R.G. (1993) DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. International Journal of Systematic Bacteriology, 43, 249–255. https://doi.org/10.1099/00207713-43-2-249
- Campos-Herrera, R., Escuer, M., Robertson, L. & Gutiérrez, C. (2006) Morphological and Ecological Characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Rioja Strain Isolated from Bibio hortulanus (Diptera: Bibionidae) in Spain. Journal of nematology, 38, 68–75.
- Daughtrey, M. & Buitenhuis, R. (2020) Integrated Pest and Disease Management in Greenhouse Ornamentals. In: Gullino, M.L., Albajes, R. & Nicot, P.C. (Eds.), Integrated Pest and Disease Management in Greenhouse Crops. Springer International Publishing, Cham, pp. 625–679. https://doi.org/10.1007/978-3-030-22304-5_22
- Dolinksi, C. & Moino-Junior, A. (2006) Utilização de nematóides entomopatogênicos nativos ou exóticos: o perigo das introduções. Nematologia Brasileira, 30 (2), 139–149.
- Dolinski, C., Kamitani, F., Machado, I. & Winter, C. (2008) Molecular and morphological characterization of heterorhabditid entomopathogenic nematodes from the tropical rainforest in Brazil. Memorias do Instituto Oswaldo Cruz, 103, 150–159. https://doi.org/10.1590/S0074-02762008000200005
- Doucet, M., Bertolotti, M., Valenzuela, M. & Sousa, G. (2000) Analysis of two isolates of a Heterorhabditis bacteriophora population detected in Córdoba, Argentina. Nematology, 2, 473–476. https://doi.org/10.1163/156854100509231
- Doucet, M.M.A.D. & Bertolotti, M.A. (1996) Una nueva población de Heterorhabditis bacteriophora Poinar 1975 (Heterorhabditidae)de Río Negro, Argentina: Caracterización y acción sobre el huésped. . Nematologia Mediterranea, 24, 69–174.
- Doucet, M.M.A.D., Doucet, M.E. & Di Rienzo, J. (1990) Análisis de la variabilidad de los caracteres morfológicos y morfométricos de Heterorhabditis bacteriophora Poinar, 1975. Nematropica, 20, 4–5.
- Eivazian Kary, N. (2010) Morphometric and Molecular characterization of new isolate of entomopathogenic nematode, Heterorhabditis bacteriophora Poinar, 1976 (Nematoda:Rhabditida) from the North of Iran. Munis Entomology and Zoology, 5, 1075–1084.
- Eivazian Kary, N., Niknam, G., Griffin, C.T., Mohammadi, S.A. & Moghaddam, M. (2009) A survey of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the north-west of Iran. Nematology, 11 (1), 107–116. https://doi.org/10.1163/156854108X398453
- Eivazian Kary, N., Rafiee Dastjerdi, H., Mohammadi, D. & Afghahi, S. (2011) Laboratory study of susceptibility of Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) to some geographical isolates of Entomopathogenic nematodes. Munis Entomology and Zoology, 5 (3), 1066–1074.
- Hominick, W.M., Briscoe, B.R., del Pino, F.G., Heng, J., Hunt, D.J., Kozodoy, E., Mracek, Z., Nguyen, K.B., Reid, A.P., Spiridonov, S., Stock, P., Sturhan, D., Waturu, C. & Yoshida, M. (1997) Biosystematics of entomopathogenic nematodes: current status, protocols and definitions. Journal of Helminthology, 71 (4), 271–298. [http://www.ncbi.nlm.nih.gov/pubmed/9443947] https://doi.org/10.1017/S0022149X00016096
- Karimi, J., Mokaram Hesar, A. & Hasani, M. (2011) Study on Native Isolate of entomopathogenic nematode, Heterorhabditis bacteriophora and its symbiont, Photorhabdus luminecens ssp. laumondii. Iranian Journal of plant protection science, 42, 353–363.
- Kaya, H.K., Aguillera, M.M., Alumai, A., Choo, H.Y., de laTorre, M., Fodor, A., Ganguly, S., Hazir, S., Lakatos, T., Pye, A., Wilson, M., Yamanaka, S., Yang, H. & Ehlers, R.U. (2006) Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biological Control, 38, 134–155. https://doi.org/10.1016/j.biocontrol.2005.11.004
- Kaya, H.K. & Gaugler, R. (1993) Entomopathogenic nematodes. Annual Review of Entomology, 38, 181–206. https://doi.org/10.1146/annurev.en.38.010193.001145
- Kaya, H.K. & Patricia Stock, S. (1997) Chapter VI—Techniques in insect nematology. In: Lacey, L. (Ed.), Manual of Techniques in Insect Pathology. Academic Press, London, pp. 281–324. https://doi.org/10.1016/B978-012432555-5/50016-6
- Kaya, H.K. & Stock, S.P. (1997) Techniques in insect nematology. In: Lacey, L. (Ed.), Manual of Techniques in Insect Pathology. Academic Press, San Diego, California, pp. 281–324.
- Kim, T.-H., Tarasco, E. & Berry, R. (2000) Pathogenicity, reproductive potential, and reproductive success of isolates of Heterorhabditis bacteriophora Poinar 1975 (Rhabditida: Heterorhabditidae) from Italy**. Entomologica, Bari, 0425-1016 (34), 33–39.
- Lewis, E.E. & Clarke, D.J. (2012) Nematode parasites and entomopathogens. In: Vega, F.E. & Kaya, H.K. (Eds.), Insect Pathology. Academic Press, San Diego, pp. 395–443. https://doi.org/10.1016/B978-0-12-384984-7.00011-7
- Manly, B.F.J. (1986) Multivariate statistical methods. A primer. Chapman & Hall, New York, New York, 159S.
- Nikdel, M., Niknam, G., Griffin, C.T. & Eivazian Kary, N. (2010) Diversity of entomopathogenic nematodes (Nematoda: Steinernematidae, Heterorhabditidae) from Arasbaran forests and rangelands in north-west Iran. Nematology, 12 (5), 767–773. https://doi.org/10.1163/138855410X12628646276168
- Parkman, J. P. & Smart, G.C.J. (1996) Entomopathogenic nematodes, a case study: introduction of Steinernema scapterisci in Florida. Biocontrol Science and Technology, 6, 413–419. https://doi.org/10.1080/09583159631389
- Phan Ke, L., Subbotin, S., Nguyen, C. & Moens, M. (2003) Heterorhabditis baujardi sp. n. (Rhabditida: Heterorhabditidae) from Vietnam and morphometric data for H. indica populations. Nematology, 5, 367–382. https://doi.org/10.1163/156854103769224368
- Poinar, G.O. & Georgis, R. (1990) Characterization and field application of Heterorhabditis bacteriophora strainHP88 (Rhabditida: Heterorhabditidae). Revue de Nematologie, 13 (4), 387–393.
- Poinar, G.O.J. (1976) Description and biology of a new insect parasitic rhabditoid, Heterorhabditis bacteriophora n. gen., n. sp. (Rhabditida: Heterorhabditidae n. fam.). Nematologica, 21 (4), 463–470. https://doi.org/10.1163/187529275X00239
- Posada, D. & Crandall, K.A. (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics, 14, 817–818. https://doi.org/10.1093/bioinformatics/14.9.817
- Qiu, L., Fang, Y., Zhou, Y., Pang, Y. & Nguyen, K.B. (2004) Steinernema guangdongense sp. n. (Nematoda:Steinernematidae), a new entomopathogenic nematode from southern China with a note on S. serratum (nomen nudum). Zootaxa, 704 (1), 1–20. https://doi.org/10.11646/zootaxa.704.1.1
- Rosa, J.S. & Simões, N. (2004) Evaluation of twenty-eight strains of Heterorhabditis bacteriophora isolated in Azores for biocontrol of the armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). Biological Control, 29 (3), 409–417. https://doi.org/10.1016/j.biocontrol.2003.07.004
- Stock, S., Griffin, C.T. & Burnell, A.M. (2002) Morphological characterisation of three isolates of Heterorhabditis Poinar, 1976 from the ‘Irish group’ (Nematoda: Rhabditida: Heterorhabditidae) and additional evidence supporting their recognition as a distinct species, H.downesi n.sp. Systematic Parasitology, 51 (2), 95–106. [http://www.ncbi.nlm.nih.gov/pubmed/11924599] https://doi.org/10.1023/A:1014062429652
- Stock, S.P. & Kaya, H.K. (1996) A multivariate analysis of morphometric characters of Heterorhabditis species (Nemata: Heterorhabditidae) and the role of morphometrics in the taxonomy of species of the genus. Journal of Parasitology, 82 (5), 806–813. [http://www.ncbi.nlm.nih.gov/pubmed/8885892] https://doi.org/10.2307/3283895
- Stock, S.P., Mrácek, Z. & Webster, J. (2000) Morphological variation between allopatric populations of Steinernema kraussei (Steiner, 1923) (Rhabditida: Steinernematidae). Nematology, 2 (2), 143–152. https://doi.org/10.1163/156854100509033
- Swofford, D.L. (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. [program]
- White, G.F. (1929) A method for obtaining infective nematode larvae from cultures. Science, 66, 302–303. https://doi.org/10.1126/science.66.1709.302.b