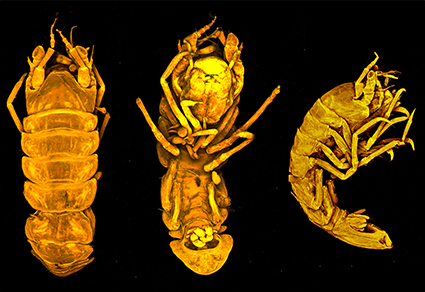
Abstract
Hadal trenches are perceived as a unique deep-sea ecosystem with fundamentally different communities compared to the nearby abyss. So far, however, scarce information exists about how populations are genetically linked within a trench and about mechanisms for species divergence. The present study presents the morphological and molecular-genetic characterization and description of a new nannoniscid species within the genus Austroniscus Vanhöffen, 1914 obtained from abyssal and hadal depths of the Puerto Rico Trench, NW Atlantic. Samples were collected as part of the Vema-TRANSIT expedition onboard RV Sonne in January 2015. Because of the large depth differences between sampling locations (4,552–8,338 m), we expected to find different species within the genus inhabiting abyssal and hadal sites. Initial morphological examination using traditional light microscopy and Confocal Laser Scanning Microscopy was paired with subsequent molecular analysis based on mtDNA (COI and 16S). Contrary to our assumptions, combined morphological and molecular species delimitation analyses (sGMYC, mPTP, ABGD) revealed the presence of only one species spanning the abyssal and hadal seafloor of the Puerto Rico Trench. In addition, comparison with type material could show that this species belongs to a new species, Austroniscus brandtae n. sp., which is described herein. Incongruence between some species delimitation methods suggesting the presence of multiple species is interpreted as strong genetic population structuring within the trench, which is also supported by the analysis of the haplotype networks. The geographic and bathymetric distribution of Austroniscus species is discussed. The species described herein represents the first in the genus Austroniscus from the Atlantic Ocean and the deepest record of the genus to date, and hence significantly expanding previously known limits of its geographic and bathymetric range.
References
- Baco, A., Etter, R., Ribeiro, P., von der Heyden, S., Beerli, P. & Kinlan, B.P. (2016) A synthesis of genetic connectivity in deep-sea fauna and implications for marine reserve design. Molecular Ecology. https://doi.org/10.1111/mec.13689
- Beliaev, G.M., & Brueggeman, P.L. (1989) Deep-Sea Ocean Trenches and Their Fauna. Nauka Publishing House, Moscow (1989), 385pp. (Translated to English by Scripps Institution of Oceanography, USA, 2004).
- Birstein, J.A. (1962) Über eine neue Art der Gattung Austroniscus (Vanhoeffen) (Crustacea, Isopoda, Asellota) aus grossen Tie-fen der Nord-Westlichen Teiles des Stillen Ozeans. Izdanija Publ Zavoda zo ribarstva, NRM 3 (2), 33–38.
- Birstein, J.A. (1970) Additions to the fauna of Isopods (Crustacea, Isopoda) of the Kurile-Kamchatka Trench. Part I. Academy of Sciences of the USSR, P.P. Shirshov Institute of Oceanology, Moscow 86 (Fauna of the Kurile-Kamchatka Trench and its Environment), 292–340.
- Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard, M.A., Rambaut, A. & Drummond, A. (2014) BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput Biol, 10 (4), e1003537. https://doi.org/10.1371/journal.pcbi.1003537
- Brandt, A., Brenke, N., Andres, H.-G., Brix, S., Guerrero-Kommritz, J., Mühlenhardt-Siegel, U. & Wägele, J.-W. (2005) Diversity of peracarid crustaceans (Malacostraca) from the abyssal plain of the Angola Basin. Organisms, Diversity and Evolution 5, 105–112. https://doi.org/10.1016/j.ode.2004.10.007
- Brandt, A, Gooday, A.J., Brandao, S.N., Brix, S., Brökeland, W., Cedhagen, T., Choudhury, M., Cornelius, N., Danis, B., De Mesel, I., Diaz, R.J., Gillan, D.C., Hilbig, B., Howe, J., Janussen, D., Kaiser, S., Linse, K., Malyutina, M., Pawlowski, J., Raupach, M. & Vanreusel, A. (2007) First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature, 447 (7142), 307. https://doi.org/10.1038/nature05827
- Brandt, A., Elsner, N., Brenke, N., Golovan, O., Malyutina, M. V., Riehl, T., Schwabe, E. & Würzberg, L. (2013) Epifauna of the Sea of Japan collected via a new epibenthic sledge equipped with camera and environmental sensor systems. Deep Sea Research Part II: Topical Studies in Oceanography, 86, 43–55. https://doi.org/10.1016/j.dsr2.2012.07.039
- Brandt, A., Brix, S., Held, C. & Kihara, T. C. (2014) Molecular differentiation in sympatry despite morphological stasis: deep-sea Atlantoserolis Wägele, 1994 and Glabroserolis Menzies, 1962 from the south-west Atlantic (Crustacea: Isopoda: Serolidae). Zoological Journal of the Linnean Society, 172 (2), 318–359. https://doi.org/10.1111/zoj.12178
- Brandt, A., Frutos, I., Bober, S., Brix, S., Brenke, N., Guggolz, T., Heitland, N., Malyutina, M., Minzlaff, M., Riehl, R., Schwabe, E., Zinkann, A.N. & Linse, K. (2018) Composition of abyssal macrofauna along the Vema Fracture Zone and the hadal Puerto Rico Trench, northern tropical Atlantic. Deep Sea Research Part II: Topical Studies in Oceanography, 148, 35–44. https://doi.org/10.1016/j.dsr2.2017.07.014
- Brix, S., Riehl, T. & Leese, F. (2011) First genetic data for species of the genus Haploniscus Richardson, 1908 (Isopoda: Asellota: Haploniscidae) from neighbouring deep-sea basins in the South Atlantic. Zootaxa, 2838 (1), 79–84. https://doi.org/10.11646/zootaxa.2838.1.5
- Brix, S., Leese, F., Riehl, T. & Kihara, T.C. (2015) A new genus and new species of Desmosomatidae Sars, 1897 (Isopoda) from the eastern South Atlantic abyss described by means of integrative taxonomy. Marine Biodiversity, 45 (1), 7–61. https://doi.org/10.1007/s12526-014-0218-3
- Brix, S., Bober, S., Tschesche, C., Kihara, T. C., Driskell, A. & Jennings, R.M. (2018) Molecular species delimitation and its implications for species descriptions using desmosomatid and nannoniscid isopods from the VEMA fracture zone as example taxa. Deep Sea Research Part II: Topical Studies in Oceanography, 148, 180–207. https://doi.org/10.1016/j.dsr2.2018.02.004
- Brix, S., Osborn, K.J., Kaiser, S., Truskey, S.B., Schnurr, S.M., Brenke, N., Malyutina, M. & Martinez Arbizu, P. (2020a) Adult life strategy affects distribution patterns in abyssal isopods–implications for conservation in Pacific nodule areas. Biogeosciences, 17 (23), 6163–6184. https://doi.org/10.5194/bg-17-6163-2020
- Brix, S., Taylor, J., Le Saout, M., Mercado-Salas, N.F., Kaiser, S., Lörz, A.-N., Gatzemeier, N., Jeskulke, K., Kürzel, K., Neuhaus, J., Paulus, E., Uhlir, C., Korfhage, S., Bruhn, M., Stein, T., Wilsenack, M., Siegler, V., Schumacher, M., Lux, T., Gärtner, L., Abegg, F., Pieper, M., Bodendorfer, M., Cuno, P., Huusmann, H., Matthiessen, T., Bischof, F. & Suck, I. (2020b) Depth transects and connectivity along gradients in the North Atlantic and Nordic Seas in the frame of the IceAGE project (Icelandic marine Animals: Genetics and Ecology). SONNE -Berichte, Cruise SO276 (MerMet17-06), Emden – Emden, [22.06.2020 – 26.07.2020]
- Brix, S., Held, C., Kaiser, S., Jennings, R.M., Driskell, A. & Brandt, A. (2021) Evolution and phylogeny of the deep-sea isopod families Desmosomatidae Sars, 1897 and Nannoniscidae Hansen, 1916 (Isopoda: Asellota). Organisms Diversity & Evolution, 1–27. https://doi.org/10.1007/s13127-021-00509-9
- Brown, A. & Thatje, S. (2011) Respiratory response of the deep-sea amphipod Stephonyx biscayensis indicates bathymetric range limitation by temperature and hydrostatic pressure. PLoS One, 6 (12), e28562. https://doi.org/10.1371/journal.pone.0028562
- Devey, C.W. (ed.) & Shipboard scientific party (2015) RV SONNE Fahrtbericht / Cruise Report SO237 Vema-TRANSIT : bathymetry of the Vema-Fracture-Zone and Puerto Rico TRench and Abyssal AtlaNtic BiodiverSITy Study, Las Palmas (Spain) - Santo Domingo (Dom. Rep.) 14.12.14 - 26.01.15. GEOMAR Report, N. Ser. 023. GEOMAR Helmholtz-Zentrum für Ozeanforschung Kiel, Kiel, Germany, 130 pp.
- DOI 10.3289/GEOMAR_REP_NS_23_2015.
- Devey, C.W., Augustin, N., Brandt, A., Brenke, N., Köhler, J., Lins, L., Schmidt, C. & Yeo, I.A. (2018) Habitat characterization of the Vema fracture zone and Puerto Rico trench. Deep Sea Research Part II: Topical Studies in Oceanography, 148, 7–20. https://doi.org/10.1016/j.dsr2.2018.02.003
- Danovaro, R., Snelgrove, P.V. & Tyler, P. (2014) Challenging the paradigms of deep-sea ecology. Trends in Ecology & Evolution, 29 (8), 465–475. https://doi.org/10.1016/j.tree.2014.06.002
- Downing, A.B., Wallace, G.T. & Yancey, P.H. (2018) Organic osmolytes of amphipods from littoral to hadal zones: Increases with depth in trimethylamine N-oxide, scyllo-inositol and other potential pressure counteractants. Deep Sea Research Part I: Oceanographic Research Papers, 138, 1–10. https://doi.org/10.1016/j.dsr.2018.05.008
- Dreutter, S., Steffen, M., Arbizu, P.M. & Brandt, A. (2020) Will the “top five” deepest trenches lose one of their members?. Progress in Oceanography, 181. https://doi.org/10.1016/j.pocean.2019.102258
- Etter, R.J., Rex, M.A., Chase, M.R. & Quattro, J.M. (2005) Population differentiation decreases with depth in deep-sea bivalves. Evolution, 59, 1479–1491. https://doi.org/10.1111/j.0014-3820.2005.tb01797.x
- France, S.C., & Kocher, T.D. (1996) Geographic and bathymetric patterns of mitochondrial 16S rRNA sequence divergence among deep-sea amphipods, Eurythenes gryllus. Marine Biology, 126 (4), 633–643. https://doi.org/10.1007/BF00351330
- Fujii, T., Kilgallen, N.M., Rowden, A.A. & Jamieson, A.J. (2013) Deep-sea amphipod community structure across abyssal to hadal depths in the Peru-Chile and Kermadec trenches. Marine Ecology Progress Series, 492, 125–138. https://doi.org/10.3354/meps10489
- Fujisawa, T., & Barraclough, T.G. (2013) Delimiting species using single-locus data and the generalized mixed yule coalescent approach: a revised method and evaluation on simulated data sets. Syst. Biol. 62, 707–724. https://doi.org/10.1093/sysbio/syt033
- Glazier, A. E., & Etter, R. J. (2014) Cryptic speciation along a bathymetric gradient. Biological Journal of the Linnean Society, 113 (4), 897–913. https://doi.org/10.1111/bij.12389
- Golovan, O.A., Blazewicz-Paszkowycz, M., Brandt, A., Budnikova, L.L., Elsner, N.O., Ivin, V.V., Lavrenteva, A.V., Malyutina, M.V., Petryashov, V.V., Tzareva L.A. (2013) Diversity and distribution of peracarid crustaceans (Malacostraca) from the continental slope and the deep-sea basin of the Sea of Japan. Deep-Sea Research II, 66–78. https://doi.org/10.1016/j.dsr2.2012.08.002
- Golovan, O., Błażewicz, M., Brandt, A., Jażdżewska, A., Jóźwiak, P., Lavrenteva, A.V., Malyutina, M.V., Petryashov, V., Riehl, T. (2018a) Diversity and distribution of peracarid crustaceans (Malacostraca) from the abyss adjacent to the Kuril-Kamchatka Trench. Marine Biodiversity, 49 (3), 1343–1360. https://doi.org/10.1007/s12526-018-0908-3
- Golovan, O. A., Malyutina, M.V. & Brandt, A. (2018b) First record of the deep-sea isopod family Dendrotionidae (Isopoda: Asellota) from the Northwest Pacific with description of two new species of Dendromunna. Marine Biodiversity, 48 (1), 531–544. https://doi.org/10.1007/s12526-017-0725-0
- Grilli, S.T., Dubosq, S., Pophet, N., Pérignon, Y., Kirby, J.T. & Shi, F. (2010) Numerical simulation and first-order hazard analysis of large co-seismic tsunamis generated in the Puerto Rico trench: near-field impact on the North shore of Puerto Rico and far-field impact on the US East Coast. Natural Hazards and Earth System Sciences, 10 (10), 2109. https://doi.org/10.5194/nhess-10-2109-2010
- Gurjanova, E. (1950) K faune ravonogich rakov (Isopoda) Tichogo okeana V. Isopod po sboram Kamchatskoi morskoi stasii Gosudarsevennogo gidrologischskogo in-ta. Akademiya Nauk SSSR. Zoologischskii Instituta, Isseldovaniia dal Nevostochnykh Morei SSSR, 2, 281–292.
- Hansen, H.J. (1916) Crustacea Malacostraca. III. V. The order Isopoda. The Danish Ingolf–Expedition. 3(5): iii+ 262 pp.
- Hessler, R.R. (1970). The Desmosomatidae (Isopoda, Asellota) of the Gay Head-Bermuda Transect. Bulletin of the Scripps Institution of Oceanography, 15, 1–185.
- Hessler, R.R., Wilson, G.D. & Thistle, D. (1979) The deep-sea isopods: a biogeographic and phylogenetic overview. Sarsia, 64 (1–2), 67–75. https://doi.org/10.1080/00364827.1979.10411365
- Jamieson, A.J. (2015) The hadal zone: Life in the deepest oceans. Cambridge University Press, 602 Cambridge, UK https://doi.org/10.1017/CBO9781139061384
- Jamieson, A.J., Fujii, T., Mayor, D.J., Solan, M. & Priede, I.G. (2010) Hadal trenches: the ecology of the deepest places on Earth. Trends in Ecology & Evolution, 25 (3), 190–197. https://doi.org/10.1016/j.tree.2009.09.009
- Jamieson, A.J., Fujii, T. (2011) Trench connection. Biology Letters, 7, 641–643. https://doi.org/10.1098/rsbl.2011.0231
- Jamieson, A.J., Kilgallen, N.M., Rowden, A.A., Fujii, T., Horton, T., Lörz, A.N., Kitazawa, K. & Priede, I.G. (2011) Bait-attending fauna of the Kermadec trench, SW Pacific Ocean: evidence for an ecotone across the abyssal-hadal transition zone. Deep Sea Research Part I: Oceanographic Research Papers, 58, 49–62. https://doi.org/10.1016/j.dsr.2010.11.003
- Jennings, R.M., Etter, R.J., & Ficarra, L. (2013) Population differentiation and species formation in the deep sea: the potential role of environmental gradients and depth. PLoS One, 8 (10), e77594. https://doi.org/10.1371/journal.pone.0077594
- Jennings, R.M., Brix, S., Bober, S., Svavarsson, J. & Driskell, A. (2018) More diverse than expected: distributional patterns of Oecidiobranchus Hessler, 1970 (Isopoda, Asellota) on the Greenland-Iceland-Faeroe Ridge based on molecular markers. Marine Biodiversity, 48 (2), 845–857. https://doi.org/10.1007/s12526-018-0857-x
- Jennings, R.M., Golovan, O. & Brix, S. (2020) Integrative species delimitation of desmosomatid and nannoniscid isopods from the Kuril-Kamchatka trench, with description of a hadal species. Progress in Oceanography, 182, 102236. https://doi.org/10.1016/j.pocean.2019.102236
- Jumars, P.A., & Hessler, R.R. (1976) Hadal community structure: implications from the Aleutian Trench. Journal of Marine Research, 34(4), 547–560.
- Kaiser, S., & Brandt, A. (2007) Two new species of the genus Austroniscus Vanhoeffen, 1914 (Isopoda: Asellota: Nannoniscidae) from the Antarctic shelf. Zootaxa, 1394(1), 47–68. https://doi.org/10.11646/zootaxa.1394.1.3
- Kaiser, S., Brix, S., Kihara, T.C., Janssen, A. & Jennings, R.M. (2018) Integrative species delimitation in the deep-sea genus Thaumastosoma Hessler, 1970 (Isopoda, Asellota, Nannoniscidae) reveals a new genus and species from the Atlantic and central Pacific abyss. Deep Sea Research Part II: Topical Studies in Oceanography, 148, 151–179. https://doi.org/10.1016/j.dsr2.2017.05.006
- Kaiser, S., Kihara, T.C., Brix, S., Mohrbeck, I., Janssen, A. & Jennings, R. (2021) Species boundaries and phylogeographic patterns in new species of Nannoniscus G.O. Sars, 1870 (Janiroidea, Nannoniscidae) from the equatorial Pacific nodule province inferred from mtDNA and morphology. Zoological Journal of the Linnean Society, 193, 3, 1020–1071, https://doi.org/10.1093/zoolinnean/zlaa174
- Kapli, P., Lutteropp, S., Zhang, J., Kobert, K., Pavlidis, P., Stamatakis, A. & Flouri, T. (2017) Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics, 33 (11), 1630–1638. https://doi.org/10.1093/bioinformatics/btx025
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30 (4), 772–780. https://doi.org/10.1093/molbev/mst010
- Kekkonen, M. & Hebert, P.D. (2014) DNA barcode-based delineation of putative species: efficient start for taxonomic workflows. Molecular Ecology Resources, 14 (4), 706–715. https://doi.org/10.1111/1755-0998.12233
- Kihara, T.C. & Rocha, C. (2009) Técnicas para o estudo taxonômico de copépodes harpacticóides da meiofauna marinha. Porto Alegre: Asterisco.
- Kniesz, K., Brandt, A. & Riehl, T. (2018) Peritrich epibionts on the hadal isopod species Macrostylis marionae n. sp. from the Puerto Rico Trench used as indicator for sex-specific behaviour. Deep Sea Research Part II: Topical Studies in Oceanography, 148, 105–129. https://doi.org/10.1016/j.dsr2.2017.10.007
- Kussakin, O.G. (1973) Peculiarities of the geographical and vertical distribution of marine isopods and the problem of deep-sea fauna origin. Marine Biology, 23 (1), 19–34. https://doi.org/10.1007/BF00394108
- Lacey, N.C., Mayor, D.J., Linley, T.D. & Jamieson, A.J. (2018) Population structure of the hadal amphipod Bathycallisoma (Scopelocheirus) schellenbergi in the Kermadec Trench and New Hebrides Trench, SW Pacific. Deep Sea Research Part II: Topical Studies in Oceanography, 155, 50–60. https://doi.org/10.1016/j.dsr2.2017.05.001
- Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., Thompson, J.D., Gibson, T.J. & Higgins, D.G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics, 23 (21), 2947–2948. https://doi.org/10.1093/bioinformatics/btm404
- Leigh, J.W., & Bryant, D. (2015) Popart: full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6 (9), 1110–1116. https://doi.org/10.1111/2041-210X.12410
- Liu, R., Wang, L., Wei, Y. & Fang, J. (2018) The hadal biosphere: Recent insights and new directions. Deep Sea Research Part II: Topical Studies in Oceanography, 155, 11–18. https://doi.org/10.1016/j.dsr2.2017.04.015
- Lörz, A.N., Jażdżewska, A.M. & Brandt, A. (2018) A new predator connecting the abyssal with the hadal in the Kuril-Kamchatka Trench, NW Pacific. PeerJ, 6, e4887. https://doi.org/10.7717/peerj.4887
- McClain, C.R. & Hardy, S.M. (2010) The dynamics of biogeographic ranges in the deep sea. Proceedings of the Royal Society B: Biological Sciences, 277(1700), 3533–3546. https://doi.org/10.1098/rspb.2010.1057
- McCallum, A. & Riehl, T. (2020) Intertidal to Abyss: Crustaceans and Depth. In Evolution and Biogeography— The Natural History of the Crustacea (Vol. 8, pp. 429–449). Oxford University Press. [https://global.oup.com/academic/product/the-natural-history-of-the-crustacea9780190637842?lang=en&cc=de#]
- Menzies, R.J. & George, R.Y. (1972) Isopod Crustacea of the Peru-Chile Trench. Anton Brunn Rep. 9, 1–124.
- Michels, J. & Büntzow, M. (2010) Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. Journal of Microscopy, 238 (2), 95–101. https://doi.org/10.1111/j.1365-2818.2009.03360.x
- Nunoura, T., Takaki, Y., Hirai, M., Shimamur, S., Makabe, A., Koide, O., Kikuchie, T., Miyazaki, J., Koba, K., Yoshida, N., Sunamura, M. & Takai, K. (2015) Hadal biosphere: insight into the microbial ecosystem in the deepest ocean on Earth. Proceedings of the National Academy of Sciences of the United States of America, 112 (11), E1230–E1236. https://doi.org/10.1073/pnas.1421816112
- Ólafsdóttir S.H. & Svavarsson, J. (2002) Ciliate (Protozoa) epibionts of deep-water asellote isopods (Crustacea): pattern and diversity. Journal of Crustacean Biology, 22 (3), 607–618. https://doi.org/10.1163/20021975-99990273
- Pentinsaari, M., Vos, R. & Mutanen, M. (2017) Algorithmic single-locus species delimitation: effects of sampling effort, variation and nonmonophyly in four methods and 1870 species of beetles. Molecular Ecology Resources, 17 (3), 393–404. https://doi.org/10.1111/1755-0998.12557
- Pons, J., Barraclough, T.G., Gomez-Zurita, J., Cardoso, A., Duran, D.P., Hazell, S., Kamoun, S., Sumlin, W.D. & Vogler, A.P. (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology, 55 (4), 595–609. https://doi.org/10.1080/10635150600852011
- Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G.J.M.E. (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology, 21 (8), 1864–1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x
- QGIS Development Team (2020) QGIS Geographic Information System (Version 3.4.7-Madeira): Open Source Geospatial Foundation Project. Available from: http://qgis.osgeo.org. (accessed 1 March 2023)
- Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. (2014) Tracer v1. 6. available from http. beast. bio. ed. ac. uk/Tracer.
- Raupach, M.J. & Wägele, J.W. (2006) Distinguishing cryptic species in Antarctic Asellota (Crustacea: Isopoda)-a preliminary study of mitochondrial DNA in Acanthaspidia drygalskii. Antarctic Science, 18 (2), 191–198. https://doi.org/10.1017/S0954102006000228
- Raupach, M.J., Mayer, C., Malyutina, M. & Wägele, J.W. (2009) Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proceedings of the Royal Society B: Biological Sciences, 276 (1658), 799–808. https://doi.org/10.1098/rspb.2008.1063
- Rex, M.A., & Etter, R.J. (2010) Deep-sea biodiversity: pattern and scale. Harvard University Press.
- Reid, N.M. & Carstens, B.C. (2012) Phylogenetic estimation error can decrease the accuracy of species delimitation: a Bayesian implementation of the general mixed Yule-coalescent model. BMC Evolutionary Biology, 12 (1), 1–11. https://doi.org/10.1186/1471-2148-12-196
- Riehl, T. & Brandt, A. (2010) Descriptions of two new species in the genus Macrostylis Sars, 1864 (Isopoda, Asellota, Macrostylidae) from the Weddell Sea (Southern Ocean), with a synonymisation of the genus Desmostylis Brandt, 1992 with Macrostylis. ZooKeys, (57), 9–49. https://doi.org/10.3897/zookeys.57.310
- Riehl, T. & Kaiser, S. (2012) Conquered from the deep sea? A new deep-sea isopod species from the Antarctic shelf shows pattern of recent colonization. PLoS One, 7 (11), e49354. https://doi.org/10.1371/journal.pone.0049354
- Riehl, T., Wilson, G.D.F. & Hessler, R.R. (2012) New Macrostylidae Hansen, 1916 (Crustacea: Isopoda) from the Gay Head-Bermuda transect with special consideration of sexual dimorphism. Zootaxa, 3277 (1), 1–26. https://doi.org/10.11646/zootaxa.3277.1.1
- Riehl, T., Brenke, N., Brix, S., Driskell, A., Kaiser, S., & Brandt, A. (2014) Field and laboratory methods for DNA studies on deep-sea isopod crustaceans. Polish Polar Research, 203–224. https://doi.org/10.2478/popore-2014-0018
- Riehl, T., Kaiser, S. & Brandt, A. (2018a) Vema-TRANSIT–An interdisciplinary study on the bathymetry of the Vema-Fracture Zone and Puerto Rico Trench as well as abyssal Atlantic biodiversity. Deep Sea Research Part II: Topical Studies in Oceanography, 148, 1–6. https://doi.org/10.1016/j.dsr2.2018.01.007
- Riehl, T., Lins, L. & Brandt, A. (2018b) The effects of depth, distance, and the Mid-Atlantic Ridge on genetic differentiation of abyssal and hadal isopods (Macrostylidae). Deep Sea Research Part II: Topical Studies in Oceanography, 148, 74–90. https://doi.org/10.1016/j.dsr2.2017.10.005
- Ritchie, H., Jamieson, A.J. & Piertney, S.B. (2017) Population genetic structure of two congeneric deep-sea amphipod species from geographically isolated hadal trenches in the Pacific Ocean. Deep Sea Research Part I: Oceanographic Research Papers, 119, 50–57. https://doi.org/10.1016/j.dsr.2016.11.006
- Sands, C. J., O’Hara, T.D. & Martín-Ledo, R. (2021) Pragmatic assignment of species groups based on primary species hypotheses: the case of a dominant component of the Southern Ocean benthic fauna. Frontiers in Marine Science, 1371. https://doi.org/10.3389/fmars.2021.723328
- Schiecke, U. & Modigh-Tota, M. (1976) Erstfund eines Vertreters der Nannoniscidae (Isopoda: Asellota) im Mittelmeer: Austroniscus coronatus n. sp. aus dem Golf von Neapel. Pubblicazioni della Stazione Zoologico di Napoli 40 (1), 105–113.
- Schnurr, S., Osborn, K.J., Malyutina, M., Jennings, R., Brix, S., Driskell, A., Svavarsson, J. & Martinez Arbizu, P. (2018) Hidden diversity in two species complexes of munnopsid isopods (Crustacea) at the transition between the northernmost North Atlantic and the Nordic Seas. Marine Biodiversity, 48 (2), 813–843. https://doi.org/10.1007/s12526-018-0877-6
- Siebenaller, J.F. & Hessler, R.R. (1977) The Nannoniscidae (Isopoda, Asellota): Hebefustis n. gen. and Nannoniscoides Hansen. Transactions of the San Diego Society of Natural History 19, 17–44.
- Siebenaller J.F. & Hessler R.R. (1981) The genera of the Nannoniscidae (Isopoda, Asellota). Trans. San Diego Soc. Nat. Hist., 19, 227–250.
- Stewart, H.A. & Jamieson, A.J. (2018) Habitat heterogeneity of hadal trenches: considerations and implications for future studies. Progress in Oceanography, 161, 47–65. https://doi.org/10.1016/j.pocean.2018.01.007
- Stewart, H.A. & Jamieson, A.J. (2019) The five deeps: The location and depth of the deepest place in each of the world’s oceans. Earth-Science Reviews, 197, 102896. https://doi.org/10.1016/j.earscirev.2019.102896
- Svavarsson, J. (1982) Nannoniscus profundus sp. n. and Austroniscus norbi sp. n. (Isopoda, Asellota, Nannoniscidae) from the deep Norwegian Sea. Sarsia 67, 179–186. https://doi.org/10.1080/00364827.1982.10420545
- Svavarsson, J., Stromberg, J.O. & Brattegard, T. (1993) The deep-sea asellote (Isopoda, Crustacea) fauna of the Northern Seas: species composition, distributional patterns and origin. Journal of Biogeography, 537–555. https://doi.org/10.2307/2845725
- Talavera, G. & Castresana, J. (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56 (4), 564–577. https://doi.org/10.1080/10635150701472164
- Taylor, M.L. & Roterman, C.N. (2017) Invertebrate population genetics across Earth’s largest habitat: The deep‐sea floor. Molecular Ecology, 26 (19), 4872–4896. https://doi.org/10.1111/mec.14237
- van Haren, H. & Gostiaux, L. (2016) Convective mixing by internal waves in the Puerto Rico Trench. Journal of Marine Research, 74 (3), 161–173. https://doi.org/10.1357/002224016819594809
- van Haren, H. (2017) AABW-transport variation and its effect on internal wave motions between top and bottom of the Puerto Rico Trench. Journal of Marine Research, 75 (4), 507–529. https://doi.org/10.1357/002224017821836716
- Vanhöffen, E. (1914) Die Isopoden der deutschen Südpolar-Expedition 1901-1903. Deutsche Südpolar-Expedition, Zoologie,15, 447–598. https://doi.org/10.5962/bhl.title.10649
- Vinogradova, N.G. (1997) Zoogeography of the abyssal and hadal zones. In: Advances in Marine Biology (Vol. 32, pp. 325–387). Academic Press. https://doi.org/10.1016/S0065-2881(08)60019-X
- Weston, J.N., Espinosa-Leal, L., Wainwright, J.A., Stewart, E.C., González, C.E., Linley, T.D., Reid, W.D.K., Hidalgo P., Oliva, M.E., Ulloa, O., Wenzhöfer, F., Glud, R., Escribano, R. & Jamieson, A.J. (2021) Eurythenes atacamensis sp. nov. (Crustacea: Amphipoda) exhibits ontogenetic vertical stratification across abyssal and hadal depths in the Atacama Trench, eastern South Pacific Ocean. Marine Biodiversity, 51 (3), 1–20. https://doi.org/10.1007/s12526-021-01182-z
- Wilson, G. D. (1998) Historical influences on deep-sea isopod diversity in the Atlantic Ocean. Deep Sea Research Part II: Topical Studies in Oceanography, 45 (1-3), 279–301. https://doi.org/10.1016/S0967-0645(97)00046-5
- Wilson, G.D. (2008) A review of taxonomic concepts in the Nannoniscidae (Isopoda, Asellota), with a key to the genera and a description of Nannoniscus oblongus Sars. Zootaxa, 1680 (1), 1–24. https://doi.org/10.11646/zootaxa.1680.1.1
- Wolff, T. (1956) Isopoda from depths exceeding 6000 meters. Galathea 2 Report 2, 85–158.
- Wolff, T. (1959) The hadal community, an introduction. Deep Sea Research 6, 95–124. https://doi.org/10.1016/0146-6313(59)90063-2
- Wolff, T. (1962) The Systematics and Biology of Bathyal and Abyssal Isopoda Asellota. Danish Science Press, Copenhagen, pp. 7–320.
- Wolff, T. (1970) The concept of the hadal or ultra-abyssal fauna. In: Deep Sea Research and Oceanographic Abstracts, Elsevier, Vol. 17, No. 6, pp. 983–1003 https://doi.org/10.1016/0011-7471(70)90049-5
- Wolff, T. (1979) Macrofaunal utilization of plant remains in the deep sea. Sarsia, 64 (1-2), 117–143. https://doi.org/10.1080/00364827.1979.10411373
- WoRMS Editorial Board (2021) World Register of Marine Species. Available from: http://www.marinespecies.org at VLIZ. (Accessed 20 October 2022)