Abstract
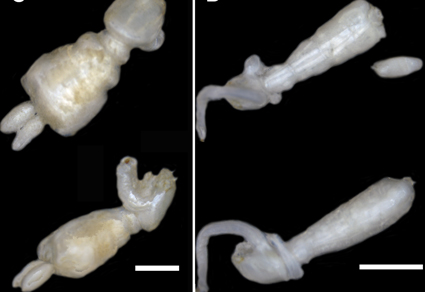
The family Lernaeopodidae includes 14 genera parasitizing elasmobranchs. Fourteen species of this family have been cited from Argentina, four of which were found on chondrichthyans. Schroederichthys bivius Müller and Henle and Galeorhinus galeus (Linnaeus) from Argentina harbored parasitic copepods of the genus Lernaeopoda. The objective of this study was to identify the species using an integrative approach. The morphology was examined by Optical Microscopy and Scanning Electron Microscopy and the molecular analysis was based on partial sequences of the COI mtDNA gene. Despite differences in the antenna, antennule, mandible, maxilliped and maxillae among the specimens, the morphological and molecular analyses revealed that they belonged to Lernaeopoda bivia Leigh-Sharpe, 1930. The species so far reported for Argentina are L. bivia and L. galei Krøyer, 1837, which are distinguished by the size (less and greater than 7 mm, respectively). Here, we report partial sequences of the COI mtDNA gene of L. bivia for the first time, obtained from eleven specimens attached to the mouth, fins, anal slit and claspers of the two shark hosts. The COI mtDNA gene tree shows that the Lernaeopoda group forms a sister clade with Pseudocharopinus bicaudatus (Krøyer, 1837), while the genus Pseudocharopinus does not appear to be a natural group. We propose that the material described from Argentinean waters as L. galei was misidentified and actually belongs to L. bivia. The wide variability within the specimens of L. bivia emphasizes the importance of using an integrative approach to revise the taxonomy of the Lernaeopoda species from all over the world.
References
- Alarcos, A.A. & Etchegoin, J.A. (2010) Parasite assemblages of estuarine-dependent marine fishes from Mar Chiquita coastal lagoon (Buenos Aires Province, Argentina). Parasitology Research, 107, 1083–1091. https://doi.org/10.1007/s00436-010-1974-z
- Boxshall, G.A. (1998) Host specificity in copepod parasites of Deep-sea fishes. Journal of Marine Systems, 15 (1–4), 215–223. https://doi.org/10.1016/S0924-7963(97)00058-4
- Brooks, D. (1979) Testing the context and extent of host-parasite coevolution. Systematic Biology, 28 (3), 299–307. https://doi.org/10.1093/sysbio/28.3.299
- Cantatore D., Braicovich P.E., Alarcos, A.J., Lanfranchi, A.L., Rossin, M.A., Vales, D.G. & Timi, J.T. (2012) New records of parasitic copepods (Crustacea, Copepoda) from marine fishes in the Argentinean Sea. Acta Parasitologica, 57, 83–89. https://doi.org/10.2478/s11686-012-0003-z
- Castro Romero, R. & Baeza Kuroki, H. (1986) Lernaeopoda tenuis n. sp. and Pseudolernaeopoda caudocapta ng, n. sp. (Copepoda, Lernaeopodidae) parasitic on Triakis maculata (Kner & Steindachner) from the Chilean coast, South Pacific. Systematic Parasitology, 8, 227–233. https://doi.org/10.1007/BF00009891
- Castro Romero, R. & Baeza Kuroki, H. (1987) On two members of the family Lernaeopodidae (Crustacea: Copepoda) parasitic on Chilean waters, with a description of Pseudocharopinoides myliobatidos n. g., n. sp. from Myliobatis chilensis. Systematic Parasitology, 9, 235–240. https://doi.org/10.1007/BF00010859
- Castro Romero, R. & González, M.T. (2005) Clavellotis sebastidis sp. nov. (Copepoda, Lernaeopodidae) parasitic on Sebastes oculatus Valenciennes, 1833 from Argentina. Acta Parasitologica, 50 (1), 74–79.
- Castro Romero, R., Montes, M.M. & Martorelli, S. (2022) Maxiclavella and Praeclavella (Siphonostomatoida: Lernaeopodidae) new genera confirmed by molecular and morphological evidence. Anais da Academia Brasileira de Ciências, 94 (4), 1–20. https://doi.org/10.1590/0001-3765202220200992.
- Castro Romero, R., Montes, M.M., Martorelli, S.R., Sepúlveda, D., Tapia, S. & Martinez Aquino, A. (2016) Integrative taxonomy of Peniculus, Metapeniculus, and Trifur (Siphonostomatoida: Pennellidae), copepod parasites of marine fishes from Chile: species delimitation analyses using DNA barcoding and morphological evidence. Systematics and Biodiversity, 14, 466–483. https://doi.org/10.1080/14772000.2016.1158213
- Dippenaar, S.M. (2020) Lernaeopoda species (Lernaeopodidae: Siphonostomatoida) from South Africa with the redescription of Lernaeopoda musteli Thomson, 1890. Marine Biodiversity, 50, 21. https://doi.org/10.1007/s12526-020-01044-0
- Dippenaar, S.M. & Kalman Passarelli, J. (2020) A redescription of the female of Lernaeopoda bivia Leigh- Sharpe, 1930 (Lernaeopodidae: Siphonostomatoida) and a first description of the male. Systematic Parasitology, 97, 675–679. https://doi.org/10.1007/s11230-020-09933-5
- Dippenaar, S.M., Narváez, K,, Osaer, F. & Mangena, T. (2021) Symbiotic Siphonostomatoida (Copepoda) of the hammerhead shark species Sphyrna zygaena (Carcharhiniformes: Sphyrnidae) and stingray Dasyatis pastinaca (Myliobatiformes: Dasyatidae) off the Canary Islands, with a re-description of Pseudocharopinus pillaii Kabata, 1979. Parasitology Research, 120, 3739–3747. https://doi.org/10.1007/s00436-021-07332-3
- Eiras, J. & Castro, R. (2016) Crustacea. In Eiras, J., Velloso, A. & Pereira, J. (Eds.), Parasitos de peixes marinhos da América do Sul. 1st Edition. Editora da furg - Universidade Federal do Rio Grande-Furg, Rio Grande, pp. 287–359.
- Etchegoin, J.A. & Ivanov, V, (1999) Parasitic copepods of the narrownose smooth-hound shark Mustelus schmitti (Chondrichthyes: Triakidae) from Argentina. Folia Parasitologica, 46, 99.
- Etchegoin, J.A., Timi, J.T. & Lanfranchi, A.L. (2006) Redescription of Neobrachiella spinicephala (Ringuelet, 1945) parasitic on Pinguipes brasilianus Cuvier, 1829 from Argentina, with the first description of the male. Acta Parasitologica, 51, https://doi.org/10.2478/s11686-006-0044-2
- Folmer, O., Black, N., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplifications of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
- González, M.T., Castro, R., Muñoz, G. & López, Z. (2016) Sea lice (Siphonostomatoida: Caligidae) diversity on littoral fishes from the south-eastern Pacific coast determined from morphology and molecular analysis, with description of a new species (Lepeophtheirus confusum). Parasitology International, 65, 685–695. https://doi.org/10.1016/j.parint.2016.08.006
- Hogans, W.E. (1988) Pseudolernaeopodina synaphobranchus n.gen.; n.sp. (Copepoda: Lernaeopodidae) from the eye of Synapobranchus kaupi Johnston, 1862, in the Northwesten Atlantic. Journal of Parasitology, 74, 861–63. https://doi.org/10.2307/3282266
- Irigoitia, M.M., Cantatore, D.M.P., Incorvaia, I.S. & Timi, J.T. (2016) Parasitic copepods infesting the olfactory sacs of skates from the southwestern Atlantic with the description of a new species of Kroeyerina Wilson, 1932. Zootaxa, 4174 (1), 137–152. https://doi.org/10.11646/zootaxa.4174.1.10
- Irigoitia, M.M., Incorvaia, I.S. & Timi, J.T. (2017) Evaluating the usefulness of natural tags for host population structure in chondrichthyans: Parasite assemblages of Sympterygia bonapartii (Rajiformes: Arhynchobatidae) in the Southwestern Atlantic. Fisheries Research, 195, 80–90. https://doi.org/10.1016/j.fishres.2017.07.006
- Irigoitia, M.M., Taglioretti, V. & Timi, J.T. (2020) Dendrapta nasicola n. sp. (Copepoda: Siphonostomatoida: Lernaeopodidae) a parasite from the olfactory sacs of Bathyraja scaphiops (Norman, 1937) in the Southwestern Atlantic. Anais da Academia Brasileira de Ciências, 92, e20180933. https://doi.org/10.1590/0001-3765202020180933
- Kabata, Z. (1979) Parasitic Copepoda of British fishes. Vol. 152. The Ray Society, London, 468 pp.
- Kabata, Z. (1986) Redescriptions of and comments on four little-known Lernaeopodidae (Crustacea: Copepoda). Canadian Journal of Zoology, 64, 1852–1859. https://doi.org/10.1139/z86-276
- Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Lanfear, R., Calcott, B., Kainer, D., Mayer, C. & Stamatakis A (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evolutionary Biology, 14, 82. https://doi.org/10.1186/1471-2148-14-82
- Leigh-Sharpe, W.H. (1930) Further remarks on the genus Lernaeopoda including a description of a new species, L. bivia. Parasitology, 22, 137–142. https://doi.org/10.1017/S0031182000010970
- Montes, M.M., Castro Romero, R., Öktener, A., Balcazar, D., Reig Cardarella, G.F. & Martorelli, S.R. (2022) Molecular evidence supports the validity of three Parabrachiella species infecting mugilids. Acta Tropica, 225, 106–211. https://doi.org/10.1016/j.actatropica.2021.106211
- Montes, M.M. (2013) Estudio comparado de las comunidades parasitarias de peces de humedales costeros: una herramienta para el monitoreo de la biodiversidad y de la salud ambiental. Doctoral Thesis, Universidad Nacional de La Plata, La Plata, 362 pp.
- Montes, M.M., Castro Romero, R. & Martorelli, S.R. (2017) Morphological identification and DNA barcoding of a new species of Parabrachiella (Siphonostomatoida: Lernaeopodidae) with aspects of their intraspecific variation. Acta Tropica, 173, 34–44. https://doi.org/10.1016/j.actatropica.2017.05.025
- Morales-Serna, F.N., Crow, G.L., Montes, M.M. & González, M.T. (2019) Description of Echthrogaleus spinulus n. sp. (Copepoda: Pandaridae) parasitic on a torpedo ray from the central Pacific Ocean utilising a morphological and molecular approach. Systematic Parasitology, 96, 777–788. https://doi.org/10.1007/s11230-019-09885-5.
- Muñoz, G. & Castro, R. (2022) Two New Parasitic Copepod Species, Clavella (Lernaeopodidae) and Haemobaphes (Pennellidae), on the Nototheniid Fish Patagonotothen cornucola (Richardson, 1844) from the Strait of Magellan, Southern Chile. Acta Parasitologica, 67, 740–751. https://doi.org/10.1007/s11686-022-00517-5
- Pereira, A.N., Timi, J.T., Lanfranchi, A.L. & Luque, J.L. (2012) A new species of Colobomatus (Copepoda, Phylichthyidae) parasitic on Mullus argentinae (Perciformes, Mullidae) from South American Atlantic coast. Acta Parasitologica, 57, 323–328. https://doi.org/10.2478/s11686-012-0032-7
- Rambaut, A. (2009) FigTree. Version 1.3.1. 2006–2009. Program package. Available from: http://tree.bio.ed.ac (accessed 18 October 2022)
- Rohde, K. (1986) Differences in species diversity of Monogenea between the Pacific and Atlantic Oceans. Hydrobiologia, 137, 21–28. https://doi.org/10.1007/BF00004168
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology, 61, 536–542. https://doi.org/10.1093/sysbio/sys029
- Sardella, N.H., Etchegoin, J.A. & Martorelli, S.R. (1995) Las comunidades parasitarias de Micropogonias furnieri (Corvina) en Argentina. Boletin del Instituto Oceanografico de Venezuela Univiversidad de Oriente, 34, 41–47.
- Sardella, N.H. & Timi, J.T. (1996). Parasite communities of Merluccius hubbsi from the Argentinian-Uruguayan common fishing zone. Fisheries Research, 27, 1–3. https://doi.org/10.1016/0165-7836(95)00460-2
- Schwarz, G. (1978) Estimating the dimension of a model. Annals of Statistics, 6, 461–464. https://doi.org/10.1214/aos/1176344136
- Stadler, T. (1974) Contribución al conocimiento de los parásitos de la fauna antártica Parte I: Clavellopsis tomoi, n. sp. Un nuevo copépodo parasito de Trematomus bernacchii Boulenger (Crustacea, Lernaeopodidae en Pisces, Nototheniidae). Contribuciones del Instituto Antártico Argentino, 176, 1–11.
- Wilson, C.B. (1915) North American parasitic copepods belonging to the Lernaeopodidae, with arevision of the entire family. Proceedings of the United States National Museum, 47, 565–729. https://doi.org/10.5479/si.00963801.47-2063.565