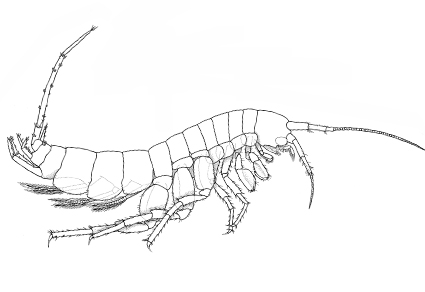
Abstract
Two new Caucasian species of the genus Niphargus Schiödte, 1849 (Crustacea: Amphipoda: Niphargidae), N. rachalechkhumensis sp. nov. and N. tvishiensis sp. nov., are described from the stygobiotic habitats of the Racha-Lechkhumi and Kvemo Svaneti karst systems in Western Georgia. Both newly described species belong to the southwestern Caucasian “Niphargus borutzkyi” ingroup related to the European “carphaticus” species complex and can be clearly separated from the congeners by morphological features, mostly in uropod III and epimeral plates, and genetically. Identification key for all known species to the “Niphargus borutzkyi” ingroup is provided, as well as their phylogenetic relationships, the estimated time of the origin and the current distribution of the ingroup in the Colchis lowland of the southwestern Caucasus are also discussed.
References
- Antić, D.Ž. & Reip, H.S. (2020) The millipede genus Leucogeorgia Verhoeff, 1930 in the Caucasus, with descriptions of eleven new species, erection of a new monotypic genus and notes on the tribe Leucogeorgiini (Diplopoda: Julida: Julidae). European Journal of Taxonomy, 713, 1–106. https://doi.org/10.5852/ejt.2020.713
- Barjadze, Sh., Asanidze, Z., Gavashelishvili, A. & Soto-Adames, F. (2019a) The hypogean invertebrate fauna of Georgia (Caucasus). Zoology in the Middle East, 65, 1–10. https://doi.org/10.1080/09397140.2018.1549789
- Barjadze, Sh., Arabuli, T., Mumladze, L., Maghradze, E., Asanidze, Z., Shavadze, L. & Gogshelidze M. (2019b) Cave Biodiversity of Georgia, Open Access Database. Available from: https://cbg.iliauni.edu.ge/en (accessed 29 November 2022)
- Birštein, J.A. (1933) Malacostraca der Kutais-Hohlen am Rion (Transkaukasus, Georgien). Zoologischer Anzeiger, 104, 143–156.
- Birštein, J.A. (1950) Cave fauna of the Western Transcaucasia. Zoologicheskiy Zhurnal, 29, 354–366. [in Russian]
- Birštein, J.A. (1961) The subterranean amphipods of the Crimea. Bulletin de la Socieìteì impeìriale des naturalistes de Moscou, 66, 126–144. [in Russian]
- Borko, Š., Trontelj, P., Seehausen, O., Moškrič, A. & Fišer, C. (2021) A subterranean adaptive radiation of amphipods in Europe. Nature Communications, 12, 3688. https://doi.org/10.1038/s41467-021-24023-w
- Borko, Š., Altermatt, F., Zagmajster, M. & Fišer, C. (2022) A hotspot of groundwater amphipod diversity on a crossroad of evolutionary radiations. Diversity & Distributions, 28, 2765–2777. https://doi.org/10.1111/ddi.13500
- Bouckaert, R., Vaughan, T.G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., De Maio, N., Matschiner, M., Mendes, F.K., Müller, N.F., Ogilvie, H.A., du Plessis, L., Popinga, A., Rambaut, A., Rasmussen, D., Siveroni, I., Suchard, M.A., Wu, Ch., Xie, D., Zhang, C., Stadler, T. & Drummond, A.J. (2019) BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 15, e1006650. https://doi.org/10.1371/journal.pcbi.1006650
- Bouckaert, R., Heled, J., Kühnert, D., Vaughan, T., Wu, Ch., Xie, D., Suchard, M.A., Rambaut, A. & Drummond, A.J. (2014) Beast 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10, e1003537. https://doi.org/10.1371/journal.pcbi.1003537
- Chertoprud, E.M., Palatov, D.M. & Vinarski, M.V. 2020. Revealing the stygobiont and crenobiont Mollusca biodiversity hotspot in Caucasus: Part II. Sitnikovia gen. nov., a new genus of stygobiont microsnails (Gastropoda: Hydrobiidae) from Georgia. Zoosystematica Rossica, 29, 258–266. https://doi.org/10.31610/zsr/2020.29.2.258
- Copilaș-Ciocianu, D. & Petrusek, A. (2015) The southwestern Carpathians as an ancient centre of diversity of freshwater gammarid amphipods: Insights from the Gammarus fossarum species complex. Molecular Ecology, 24, 3980–3992. https://doi.org/10.1111/mec.13286
- Djanashvili, R. & Barjadze, Sh. (2011) A new species of the genus Plutomurus Yosii, 1956 (Collembola, Tomoceridae) from Georgian caves. Journal of cave and karst studies, 73, 28–30. https://doi.org/10.4311/JCKS2010LSC0147
- Djanashvili, R., Barjadze, Sh., Jordana, R. & Burkhardt, U. (2014) Redefinition of the genus Argonychiurus Bagnall, 1949 (Collembola, Onychiuridae) with description of a new species from Georgia. Zootaxa, 3835 (3), 381–391. https://doi.org/10.11646/zootaxa.3835.3.8
- Fiera, C., Arbea, J.I., Vargovitsh, R.S. & Barjadze, Sh. (2021) A synthesis on troglobitic springtails in Europe. Journal of Zoological Systematics and Evolutionary Research, 59, 1874–1890. https://doi.org/10.1111/jzs.12560
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit 1 from diverse metazoan. Molecular Marine Biology and Biotechnology, 3, 294–299.
- Grego, J., Mumladze, L., Falniowski, A., Osikowski, A., Rysiewska, A., Palatov, D.M. & Hofman, S. (2020) Revealing the stygobiotic and crenobiotic molluscan biodiversity hotspot in Caucasus: Part I. The phylogeny of stygobiotic Sadlerianinae Szarowska, 2006 (Mollusca, Gastropoda, Hydrobiidae) from Georgia with descriptions of five new genera and twenty-one new species. ZooKeys, 955, 1–77. https://doi.org/10.3897/zookeys.955.51983
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010) New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Systematic Biology, 59, 307–321. https://doi.org/10.1093/sysbio/syq010
- Guy-Haim, T., Simon-Blecher, N., Frumkin, A., Naaman, I. & Achituv, Y. (2018) Multiple transgressions and slow evolution shape the phylogeographic pattern of the blind cave-dwelling shrimp Typhlocaris. PeerJ, 6, e5268. https://doi.org/10.7717/peerj.5268
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symposium Series, 41, 95–98.
- Horton, T., Lowry, J., De Broyer, C., Bellan-Santini, D., Coleman, C.O., Corbari, L., Costello, M.J., Daneliya, M., Dauvin, J.-C., Fišer, C., Gasca, R., Grabowski, M., Guerra-García, J.M., Hendrycks, E., Hughes, L., Jaume, D., Jazdzewski, K., Kim, Y.-H., King, R., Krapp-Schickel, T., LeCroy, S., Lörz, A.-N., Mamos, T., Senna, A.R., Serejo, C., Souza-Filho, J.F., Tandberg, A.H., Thomas, J.D., Thurston, M., Vader, W., Väinölä, R., Vonk, R., White, K. & Zeidler, W. (2023) World Amphipoda Database. Niphargus Schiödte, 1849. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=546804 (ccessed 31 January 2023)
- Jinjikhadze, P. & Chkheidze O. (2018) Geomorphological Zoning of Okriba Karst. Bulletin of the Georgian National Academy of Sciences, 12, 76–84.
- Karaman, G.S. (2012) New species of the subterranean genus Niphargus Schiödte, 1849 (Amphipoda, Gammaridea, Niphargidae) from Russia, N. krasnodarus sp. n. (Contribution to knowledge of Amphipoda 256). Biologia Serbica, 34, 12–16.
- Kimura, M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. https://doi.org/10.1007/BF01731581
- Kumar, S., Stecher, G., Tamura, K. (2016) MEGA 7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054
- Lanfear, R., Frandsen, P.B., Wright, A.M., Senfeld, T. & Calcott, B. (2016) PartitionFinder 2: new methods for selecting partitioned models of evolution formolecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34 (3), 772–773. https://doi.org/10.1093/molbev/msw260
- Lefébure, T., Douady, C.J., Gouy, M., Trontelj, P., Briolay, J. & Gibert J. (2006) Phylogeography of a subterranean amphipod reveals cryptic diversity and dynamic evolution in extreme environments. Molecular Ecology, 15, 1797–1806. http://doi.org/10.1111/j.1365-294X.2006.02888.x
- Marin, I. (2017) Troglocaris (Xiphocaridinella) kumistavi sp. nov., a new species of stygobiotic atyid shrimp (Crustacea: Decapoda: Atyidae) from Kumistavi Cave, Imereti, Western Georgia, Caucasus. Zootaxa, 4311 (4), 576–588. https://doi.org/10.11646/zootaxa.4311.4.9
- Marin, I.N. (2019) Crustacean “cave fishes” from the Arabika karst massif (Abkhazia, Western Caucasus): new species of stygobiotic crustacean genera Xiphocaridinella and Niphargus from the Gegskaya Cave and adjacent area. Arthropoda Selecta, 28, 225–245. https://doi.org/10.15298/arthsel.28.2.05
- Marin, I.N. (2020) The Quaternary speciation in the Caucasus: a new cryptic species of stygobiotic amphipod of the genus Niphargus (Crustacea: Amphipoda: Niphargidae) from the Kumistavi (Prometheus) Cave, Western Georgia. Arthropoda Selecta, 29, 419–432. https://doi.org/10.15298/arthsel.29.4.04
- Marin, I. & Barjadze, Sh. (2022) A new species of stygobiotic atyid shrimps of the genus Xiphocaridinella (Crustacea: Decapoda: Atyidae) from the Racha-Lechkhumi and Kvemo Svaneti, with a new record of X. kumistavi from the Imereti, Western Georgia, Caucasus. Invertebrate Zoology, 19, 24–34. https://doi.org/10.15298/invertzool.19.1.04
- Marin, I., Krylenko, S. & Palatov, D. (2021a) The Caucasian relicts: a new species of the genus Niphargus (Crustacea: Amphipoda: Niphargidae) from the Gelendzhik-Tuapse area of the Russian southwestern Caucasus. Zootaxa, 4963 (3), 483–504 https://doi.org/10.11646/zootaxa.4963.3.5
- Marin, I., Krylenko, S. & Palatov, D. (2021b) Euxinian relict amphipods of the Eastern Paratethys in the subterranean fauna of coastal habitats of the Northern Black Sea region. Invertebrate Zoology, 18, 247–320. https://doi.org/10.15298/invertzool.18.3.05
- Marin, I. & Palatov, D. (2019a) A new species of the genus Niphargus (Crustacea: Amphipoda: Niphargidae) from the southwestern part of the North Caucasus. Zoology in the Middle East, 65, 336–346. https://doi.org/10.1080/09397140.2019.1663907
- Marin, I. & Palatov, D. (2019b) An occasional record of the amplexus in epigean Niphargus (Amphipoda: Niphargidae) from the Russian Western Caucasus. Zootaxa, 4701 (1), 97–100. http://doi.org/10.11646/zootaxa.4701.1.8
- Marin, I. & Palatov, D. (2021) Cryptic refugee on the northern slope of the Greater Caucasian Ridge: Discovery of Niphargus (Crustacea: Amphipoda: Niphargidae) in the North Ossetia-Alania, North Caucasus, separated from its relatives in the late Miocene. Zoologischer Anzeiger, 292, 163–183. https://doi.org/10.1016/j.jcz.2021.03.002
- Marin, I. & Palatov, D. (2023) Insights on the Existence of Ancient Glacial Refugee in the Northern Black/Azov Sea Lowland, with the Description of the First Stygobiotic Microcrustacean Species of the Genus Niphargus Schiödte, 1849 from the Mouth of the Don River. Diversity, 15 (5), 682. https://doi.org/10.3390/d15050682
- Marin, I. & Sokolova, A. (2014) Redescription of the stygobiotic shrimp Troglocaris (Xiphocaridinella) jusbaschjani Birštein,1948 (Decapoda: Caridea: Atyidae) from Agura River, Sochi, Russia, with remarks on other representatives of the genus from Caucasus. Zootaxa, 3754 (3), 277–298. https://doi.org/10.11646/zootaxa.3754.3.3
- Marin, I. & Turbanov, I. (2021) Molecular genetic analysis of stygobiotic shrimps of the genus Xiphocaridinella (Crustacea: Decapoda: Atyidae) reveals a connection between distant caves in Central Abkhazia, southwestern Caucasus. International Journal of Speleology (Edizione Italiana), 50 (3), 301–311. https://doi.org/10.5038/1827-806X.50.3.237
- Marin, I.N., Turbanov, I., Prokopov, G. & Palatov, D. (2022) A New Species of the Genus Niphargus Schiödte, 1849 (Crustacea: Amphipoda: Niphargidae) from Groundwater Habitats of the Tarkhankut Upland, Crimean Peninsula. Diversity, 14 (12), 1010. https://doi.org/10.3390/d14121010
- Mayrs, N., Mittermeier, R., Mittermeier, C., Fonseca, G. & Kent, J. (2000) Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. https://doi.org/10.1038/35002501
- Mclnerney, C.E., Maurice, L., Robertson, A.L., Knight, L.R.F.D., Arnscheidt, J., Venditti, C., Dooley, J.S.G., Mathers, T., Matthijs, S., Eriksson, K., Proudlove, G.S. & Hänfling, B. (2014) The ancient Britons: groundwater fauna survived extreme climate change over tens of millions of years across NW Europe. Molecular Ecology, 23, 1153–1166. https://doi.org/10.1111/mec.12664
- Mittermeier, R.A., Gil, P.R., Michael Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J. & da Fonseca G.A.B. (2005) Hot spots revisited—Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions. University of Chicago Press, Chicago, 392 pp.
- Palatov, D.M. & Marin, I.N. (2021) Epigean (pond-dwelling) species of the genus Niphargus Schiödte, 1849 (Crustacea: Amphipoda: Niphargidae) from the coastal plains of the Black and Azov seas of the north- and south-western Caucasus. Invertebrate Zoology, 18, 105–151. http://doi.org/1010.15298/invertzool.18.2.05
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61 (3), 539–542. https://doi.org/10.1093/sysbio/sys029.
- Rendoša, M., Delić, T., Copilaș-Ciocianu, D. & Fišer, C. (2021) First insight into cryptic diversity of a Caucasian subterranean amphipod of the genus Niphargus (Crustacea: Amphipoda: Niphargidae). Zoologischer Anzeiger, 290, 1–11. https://doi.org/10.1016/j.jcz.2020.10.005
- Sadowsky, A.A. (1930) Xiphocaridinella kutaissiana nov. gen. et sp. (Fam. Atyidae) aus einer unterirdischen Höhle bei Kutais. Zakavkazskij kraevedstenny sbornik naucnoissledovatel’nogo kraevedstvenogo kabineta Universiteta Tiflis, 1, 93–104.
- Schiödte, J.C. (1849) Specimen faunæ subterraneae: bidrag til den ‘underjordiske fauna. Videnskabernes Selskabs Skrifter, Series 6, Raekke naturvidenskabelig Og. Mathematisk Afdeling, 1–39.
- Straškraba, M. (1972) Les groupments des especes du genre Niphargus (sensu lato). In: Ruffo, S. (Ed.), Acets du Ier Colloque Internationale sur le genre Niphargus. Museo Civico di Storia Naturale di Verona, Verona, pp. 85–90.
- Turbanov, I.S., Palatov, D.M., Golovatch, S.I. (2016) The present state of the art of biospeleology in Russia and the countries of the former Soviet Union: A review of the cave (endogean) invertebrate fauna. 1. Introduction—Crustacea. Zoologichesky Zhurnal, 95, 1136–1159. [in Russian] https://doi.org/10.7868/S0044513416100093
- Väinölä, R., Witt, J.D.S., Grabowski, M., Bradbury, J.H., Jazdzewski, K. & Sket, B. (2008) Global diversity of amphipods (Amphipoda: Crustacea) in freshwater. Hydrobiologia, 595, 241–255. https://doi.org/10.1007/s10750-007-9020-6
- Yuzbashyan, S.М. (1942) To study the fauna in Georgia. Communications of the Academy of Sciences of the Georgian SSR, 3, 1077–1084. [in Russian]
- Zazanashvili, N., Sanadiradze, G., Bukhnikashvili, A., Kandaurov, A. & Tarkhnishvili, D. (2004) Caucasus. In: Mittermeier, R.A., Robles Gil, P., Hoffmann. M., Pilgrim, J., Brooks, T., Mittermeier C.G., Lamoreux, J. & da Fonseca, G.A.B. (Eds.), Hotspots Revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. CEMEX/Agrupacion Sierra Madre, Mexico City, pp. 148–153.