Abstract
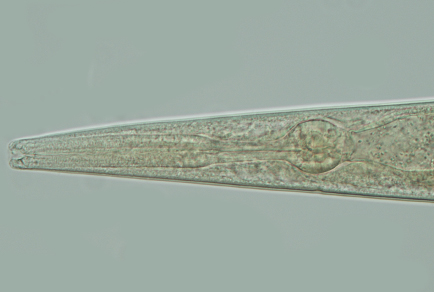
A species of genus Panagrellus was discovered from a wet season form of an oriental common evening brown butterfly Melanitis leda. In this study, a detailed description of Panagrellus ceylonensis is provided including the morphometry, light microscopy and molecular (18S and 28S rDNA genes) studies. Morphological studies on the species agree with original description and characterized by having 1250–1481µm long body in females and 949–1305µm in males, lateral fields with four longitudinal incisures, lip region continuous and 8–11 µm wide, six offset lips with protruding labial sensilla, neck 124–173 µm long, excretory pore at the level of basal bulb, vulva post-equatorial (V = 68–71), vagina anteriorly orientated with heavily muscled vaginal walls, post-vulval uterine sac 111–135 µm long or 1.7–2.6 times as long as the corresponding body diameter, tail conical elongate with an acute terminus in both sexes, spicule 71–91 µm long, ventrally curved having hooked manubrium and bifurcated lamina tip, lamina ventrally curved with dorsal deflexion at about 60% of spicule length, gubernaculum 26–31 µm long and well developed. Morphologically, the Indian population of P. ceylonensis does not show a significant difference from the type material of P. ceylonensis in the original description. For molecular studies of this species, the sequence of 18S rDNA is obtained for the first time. Phylogenetic trees based on 18S and 28S rDNA sequences are provided in this study. Additionally, bionomics and global distribution of the species of Panagrellus genus are also discussed. In conclusion, our study provides a comprehensive morphological characterisation and molecular marker sequences of 18S, and 28S genes that can be used to support future taxonomical research on this species and emphasizes the importance of combining molecular data with morphological data to describe the species accurately.
References
- Abolafia, J., Alizadeh, M. & Khakvar, R. (2016) Description of Panagrellus ulmi sp. n. (Rhabditida, Panagrolaimidae) from Iran, and comments on the species of the genus and its relatives. Zootaxa, 4162 (2), 245–267. https://doi.org/10.11646/zootaxa.4162.2.3
- Abolafia, J. & Vecchi, M. (2021) Redescription and phylogenetic analysis of the type species of the genus Panagrellus Thorne, 1938 (Rhabditida, Panagrolaimidae), P. pycnus Thorne, 1938, including the first SEM study. Journal of Nematology, 53, e2021-80. https://doi.org/10.21307/jofnem-2021-080
- Andrássy, I. (1958) Szabadonélő fonálférgek (Nematoda libera). Magyarország állatvilága, 3. Kötot, 1, Nemathelminthes Archipodiata. 1. Akadémiai Kiadó. Fauna Hungariae, 36, pp. 1–362.
- Andrássy, I. (1984) Klasse Nematoda (Ordnungen Monhysterida, Desmoscolecida, Araeolaimida, Chromadorida, Rhabditida). Bestimmungsbücher zur Bodenfauna Europas. No. 9. Deutschland, Akademie Verlag, Berlin, 509 pp. https://doi.org/10.1515/9783112484586
- Andrássy, I. (2005) Free-living nematodes of Hungary (Nematoda errantia). Vol. 1. In: Csuzdi, C. & Mahunka, S. (Eds.), Pedozoologica Hungarica. No. 3. Hungarian Natural History Museum, Budapest, pp. 1–518.
- Aubertot, M. (1925) Nématodes d’Alsace. Observations sur l’Anguillule de la bière (Anguillula silusiae de Man 1914). Bulletin de la Association Philomathique d’Alsace et de Lorraine, 6, 333–342.
- Baker, A.D. (1962) Check lists of the nematode superfamilies Dorylaimoidea, Rhabditoidea, Tylenchoidea and Aphelenchoidea. Leiden, Brill, 261 pp. https://doi.org/10.1163/9789004631779
- Brunold, E. (1950) Über eine neue Nematodenart der Gattung Anguillula aus Drosophila-Nährböden. Vierteljahrsschrift der Naturf. Gesellschaft in Zürich, 95, 148–150.
- Brunold, E. (1954) Zür Morphologie, Biologie und bakterienfreien Züchtung des Nematoden Panagrellus zymosiphilus Brunold 1950. Zeitschrift für Morphologie und Ökologie der Tiere, 42, 373–420. https://doi.org/10.1007/BF00407581
- Camerota, M., Mazza, G., Carta, L.K., Paoli, F., Torrini, G., Benvenuti, C., Carletti, B., Francardi, V. & Roversi, P.F. (2016) Occurrence of Panagrellus (Rhabditida: Panagrolaimidae) Nematodes in a Morphologically Aberrant Adult Specimen of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of nematology, 48 (1), 1–6.
- https://doi.org/10.21307/jofnem-2017-001
- Corrêa de Carvalho, J. & Álvares Corrêa, M.O. (1953) A ocorrência de nematoides em massa de tomate. Revista do Instituto Adolfo Lutz, 13, 37–44.
- Courtney, W.D., Polley, D. & Miller, V.L. (1955) TAF, an improved fixative in nematode technique. Plant Dis Reporter, 39 (7), 570–571.
- De Ley, P., De Ley, I.T., Morris, K., Abebe, E., Mundo-Ocampo, M., Yoder, M. & Thomas, W. K. (2005) An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philosophical Transactions of the Royal Society B: Biological Sciences, 360 (1462), 1945–1958. https://doi.org/10.1098/rstb.2005.1726.
- Man, J.G. de (1881) Die Eingheimischen, frei in der reinen erde und im süssen wasser lebends Nematoden. Nederlandsch Tijdschrift voor de Dierkunde, Ver. 5, 1–104.
- Félix, M.A., Ailion, M., Hsu, J.C., Richaud, A. & Wang, J. (2018) Pristionchus nematodes occur frequently in diverse rotting vegetal substrates and are not exclusively necromenic, while Panagrellus redivivoides is found specifically in rotting fruits. PLOS ONE, 13 (8), e0200851.
- https://doi.org/10.1371/journal.pone.0200851
- Goodey, T. (1943) On the systematic relationship of the vinegar eelworm, Turbatrix aceti and its congeners, with a description of a new species. Journal of Helminthology, 21, 1–9. https://doi.org/10.1017/S0022149X00031825
- Goodey, T. (1945) A note on the subfamily Turbatricinae and the genus Turbator Goodey, 1943. Journal of Helminthology, 21, 69–70. https://doi.org/10.1017/S0022149X00031953
- Goodey, T. (1963) Soil and freshwater nematodes. 2nd Edition. Rewritten by J. B. Goodey. London, Methuen & Co., 544 pp.
- Hechler, H.C. (1971a) Taxonomic notes on four species of Panagrellus Thorne (Nematoda, Cephalobidae). Journal of Nematology, 3, 227–237.
- Hechler, H.C. (1971b) Panagrellus ceylonensis n. sp. (Nematoda, Cephalobidae). Journal of Nematology, 3, 246–250.
- Heena & Chaubey, A.K. (2023) In vitro cost-effective culture technique for pure line production of facultative nematode parasites of insects and free-living nematodes. Russian journal of nematology, 31 (2), 101–109. https://doi.org/10.24412/0869-6918-2023-2-101-109
- Heindl-Mengert, H. (1956) Die Nematodenfauna im Schleimfluss lebender Laubbaüme. Sitzungberichte der Physikalisch Medizinischen Sozietät Erlangen, 77, 158–176.
- Ivanova, E., Perfilieva, K. & Spiridonov, S. (2018) Panagrellus levitatus sp. n. (Rhabditida: Panagrolaimidae), a nematode suppressing Drosophila melanogaster in laboratory cultures. Nematology, 20. https://doi.org/10.1163/15685411-00003141
- Kimura, M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16 (2), 111–120. https://doi.org/10.1007/BF01731581
- Knot, I.E., Zouganelis, G.D., Weedall, G.D., Wich, S.A. & Rae, R. (2020) DNA Barcoding of Nematodes Using the MinION. Frontiers in Ecology and Evolution, 8. https://doi.org/10.3389/fevo.2020.00100
- Linnaeus, C. (1758) Systema Naturae. Vol. 1. Regnum Animale. 10 th Edition. Laurentii Salvii, Holmiae, 824 pp.
- Linnaeus, C. (1767) Systema naturae sive regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Vol. 1. Part 2. 12th Edition, Laurentii Salvii, Holmiae, pp. 533–1327. https://doi.org/10.5962/bhl.title.158187
- Man, J.G. de (1910) Beiträge zur Kenntnis der in dem weißen Schleimfluß der Eichen lebeden Anguilluliden, nebst Untersuchungen über den Bau des Essigälchens und der Gattung Anguillula Ehrb. Zoologische Jahrbüch Systematik, 29, 359–394.
- Man, J.G. de (1913) Anguillula silusiae n. sp., eine neue in der sogenannten „Bierfilzen“ lebende Art der Gattung Anguillula Ehrb. Centralblatt für Bakteriologie, Parasitenkkunde und Infektionskrankheiten, Zweite Abteilung, 39, 73–74.
- Man, J.G. de (1914) Anguillula silusiae de Man, eine neue in der sogenannten Bierfilzen lebende Art der Gattung Anguillula Ehrb. Annales de la Sociéte Royale Zoologique et Malacologique de Belgique, 48 (Vol. Jubilaire), 1–9.
- Massey, C.L. (1974) Biology and taxonomy of nematode parasites and associates of bark beetles in the Unites States. Agriculture Handbook, 446 (Forest Service, U.S. Department of Agriculture), 1–233.
- Menzel, R. (1922) Beiträge zur kenntnis der Mikrofauna von Niederländisch-ost-Indien. Treubia, 3, 116–126.
- Micoletzky, H. & Menzel, R. (1928) Beiträge zur kenntnis der Mikrofauna von niederländisch Ost-Indien. Treubia, 10, 285–290.
- Nunn, G.B. (1992) Nematode molecular evolution. An investigation of evolutionary patterns among nematodes based upon DNA sequences. Ph.D. dissertation, University of Nottingham, 228 pp.
- Rodríguez-Martínez, R., Mendoza-de-Gives, P., Aguilar-Marcelino, L., López-Arellano, M. E., Gamboa-Angulo, M., Hanako Rosas-Saito, G., Reyes-Estébanez, M. & Guadalupe García-Rubio, V. (2018) In-vitro lethal activity of the nematophagous fungus Clonostachys rosea (Ascomycota: Hypocreales) against nematodes of five different taxa. BioMed research international, 1–7. https://doi.org/10.1155/2018/3501827
- Rühm, W. (1956) Die Nematoden der Ipiden. Parasitologische Schriftenreihe, 6, 1–437.
- Samoiloff, M.R. & Pasternak, J. (1969) Nematode morphogenesis: the structure of the molting cycles in Panagrellus silusiae (de Man 1913) Goodey 1945. Canadian Journal of Zoology, 47, 639–643. https://doi.org/10.1139/z69-109
- Sandground, J.H. (1939) Cephalobus parasiticus n. sp. and “Pseudostrongyloidiasis” in Macaca irus mordax. Parasitology, 31, 132–137. https://doi.org/10.1017/S003118200001266X
- Sanwal, K.C. (1960) Panagrellus dubius n. sp. (Nematoda, Turbatricinae Goodey, 1943) from frass of beetle Sternochetus lapathi (L.), with remarks on redescriptions of Anguillula rediviva (L., 1767). Canadian Journal of Zoology, 38, 1041-1046. https://doi.org/10.1139/z60-108
- Schenk, J., Geisen, S., Kleinboelting, N. & Traunspurger W. (2019) Metabarcoding data allow for reliable biomass estimates in the most abundant animals on earth. Metabarcoding and Metagenomics, 3: e46704. https://doi.org/10.3897/mbmg.3.46704
- Seinhorst, J.W. (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica, 4 (1), 67–69. https://doi.org/10.1163/187529259X00381
- Steiner, G. (1936) Opuscula miscellanea nematologica, III. Proceedings of the Helminthological Society of Washington, 3, 16–22.
- Stewart, G., Perry, R. & Wright, D. (2001) Occurrence of dopamine in Panagrellus redivivus and Meloidogyne incognita. Nematology, 3 (8), 843–848. https://doi.org/10.1163/156854101753625335
- Stiles, C.W. & Hassall, A. (1905) The determination of generic types and a list of round-worm genera, with their original and type species. Bulletin of Bureau of Animal Industry, U. S. Department of Agriculture, 79, 1–150. https://doi.org/10.5962/bhl.title.31560
- Stock, S.P. & Nadler, A. (2006) Morphological and molecular characterisation of Panagrellus spp. (Cephalobina: Panagrolaimidae): taxonomic status and phylogenetic relationships. Nematology, 8, 921–938. https://doi.org/10.1163/156854106779799277
- Sukul, N.C. (1971) New free-living nematodes from the thermal spring of Tantloya, Bihar. Indian Journal of Helminthology, 23, 135–145.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30 (12): 2725–2729. https://doi.org/10.1093/molbev/mst197
- Tamura, K. (1992) Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Molecular biology and evolution, 9 (4), 678–687. https://doi.org/10.1093/oxfordjournals.molbev.a040752
- Thorne, G. (1938) Notes on free-living and plant-parasitic nematodes, IV. Proceedings of the Helminthological Society of Washington, 5, 64–65.
- Tsalolikhin, S.J. (1965) Novaja saprobiotischeskaja nematoda Panagrellus silusoides sp. n. Vestnik Leningradskogo Gosudarstvennogo Universiteta, 21, 152–153.
- Yokoo, T. & Ota, Y. (1961) On the variations of dimensions within soil nematodes. III. Dimensions of the Brevibucca japonica n. sp. found in the decayed fruit of pear in Japan, with descriptions of this new species. Agricultural Bulletin of Saga University, 12, 149–156.
- Yuen, P.H. (1968) Electron microscopical studies on the anterior end of Panagrellus silusiae (Rhabditidae). Nematologica, 14, 554–564. https://doi.org/10.1163/187529268X00255
- Zullini, A. (1982) Nematodi (Nematoda). Guide per il riconoscimiento delle specie animali delle acque interne italiane, 17, 1–117.