Abstract
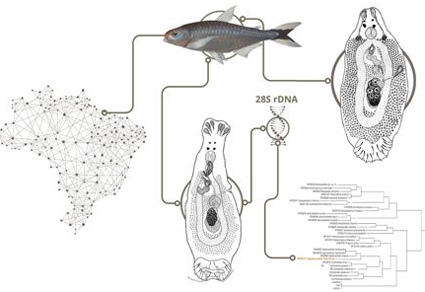
The present study integrates molecular and morphological data to support the proposal of new species of Telethecium Kritsky, Van Every & Boeger, 1996 and Diaphorocleidus Jogunoori, Kritsky & Venkatanarasaiah, 2004 from the nasal cavities of Bryconops melanurus (Bloch) of the coastal drainages of the Eastern Amazon. Telethecium tiquira sp. n. is characterized by possessing a male copulatory organ (MCO) with two circular sclerotized brims on the base, a coiled tubular shaft having 1 ½ counterclockwise rings, an accessory piece with enlarged base, pincer-shaped at the distal portion; a sclerotized calyx-shaped vaginal vestibule, and hooks with proximal shank dilatation comprising 3/4 of the shank length. Also, Telethecium tiquira sp. n. can be easily distinguished from other species of the genus by the absence of a protruding bag located at the level of the copulatory complex. Diaphorocleidus forficata sp. n. is characterized by having a MCO with two counterclockwise rings, circular sclerotized tandem brim associated with the base of the MCO; accessory piece non-articulated with the MCO, bifurcate, pincer-shaped; vaginal pore sinistral-ventral with opening marginal, vaginal canal sclerotized, elongated, comprising one loop in the proximal portion before entering to the seminal receptacle; ventral anchor with shaft elongated and evenly curved on the axis; point short and slightly curved, and hooks similar in shape and size, hooks with proximal dilatation comprising approximately ½ of the shank length. Furthermore, D. forficata sp. n. is supported by phylogenetic analysis based on sequences of the partial 28S rDNA gene, which placed D. forficata sp. n. in a well-supported clade of Diaphorocleidus spp. of characiform fishes. Thus, the two new species described here expand our knowledge about the diversity of monopisthocotylan parasites from the nasal cavities of Neotropical fishes. The findings of this study provide valuable insights into the biodiversity of the region and highlight the importance of further research in this area.
References
- Acosta, A.A., Queiroz, J., Brandão, H. & Silva, R.J. (2015) Helminth fauna of Astyanax fasciatus Cuvier, 1819, in two distinct sites of the Taquari River, São Paulo State, Brazil. Brazilian Journal of Biology, 75, 242–250. https://doi.org/10.1590/1519-6984.15113
- Acosta, A.A., Franceschini, L., Zago, A.C., Scholz, T. & Silva, R.J. (2017) Six new species of Heteropriapulus (Monogenea: Dactylogyridae) from South American fishes with an amended diagnosis to the genus. Zootaxa, 4290 (3), 459–482. https://doi.org/10.11646/zootaxa.4290.3.3
- Acosta, A.A., Scholz, T., Blasco-Costa, I., Alves, V. & Silva, R.J. (2018) A new genus and two new species of dactylogyrid monogeneans from gills of Neotropical catfishes (Siluriformes: Doradidae and Loricariidae). Parasitology International, 67, 4–12. https://doi.org/10.1016/j.parint.2017.09.012
- Acosta, A.A., Mendoza-Palmero, C.A., Silva, R.J. & Scholz, T. (2019) A new genus and four new species of dactylogyrids (Monogenea), gill parasites of pimelodid catfishes (Siluriformes: Pimelodidae) in South America and the reassignment of Urocleidoides megorchis Mizelle et Kritsky, 1969. Folia Parasitologica, 66, 1–12. https://doi.org/10.14411/fp.2019.004
- Acosta, A.A., Smit, N.J. & Da Silva, R.J. (2020) Diversity of helminth parasites of eight Siluriformes fishes from the Aguapeí River, upper Paraná basin, São Paulo state, Brazil. International Journal for Parasitology: Parasites and Wildlife, 11, 120–128. https://doi.org/10.1016/j.ijppaw.2020.01.003
- Aguiar, J.C., Ceccarelli, P.S. & Luque, J.L. (2011) Two new species of Pavanelliella (Monogenea, Dactylogyridae) parasitic on pimedolid fishes from Mogi Guaçu river, Southeastern Brazil, and notes on the morphology of P. pavanellii. Neotropical Helminthology, 5 (2), 213–224. [http://sisbib.unmsm.edu.pe/BVRevistas/neohel/v5n2/pdf/a07v5n2.pdf.pdf]
- Aguiar, J.C., Maia, A.A.M., Silva, M.R.M., Ceccarelli, P.S., Domingues, M.V. & Adriano, E.A. (2017) An integrative taxonomic study of Pavanelliella spp. (Monogenoidea, Dactylogyridae) with the description of a new species from the nasal cavities of an Amazon pimelodid catfish. Parasitology International, 66 (6), 777–788. https://doi.org/10.1016/j.parint.2017.09.003
- Betancur-R, R., Wiley, E.O., Arratia, G., Acero, A., Bailly, N., Miya, M., Lecointre, G. & Ortí, G. (2017) Phylogenetic classification of bony fishes. BMC Evolutionary Biology, 17 (162), 1–40. https://doi.org/10.1186/s12862-017-0958-3
- Boeger, W.A., Domingues, M.V. & Pavanelli, G.C. (1995) Neotropical Monogenoidea. 24. Rhinoxenus bulbovaginatus sp. n. (Dactylogyridae, Ancyocephalinae) from the nasal cavity of Salminus maxillosus (Osteichtyes, Characidae) from the Rio Paraná, Paraná, Brazil. Memórias do Instituto Oswaldo Cruz, 90 (6), 695–698. https://doi.org/10.1590/S0074-02761995000600007
- Boeger, W.A. & Vianna, R.T. (2006) Monogenoidea. In: Thatcher, V.E. (Ed.), Aquatic Biodiversity in Latin America. Amazon fish parasites. 2nd Edition. Pensoft Publishers, Sofia-Moscow, pp. 42–116.
- Bush, A.O., Lafferty, K.D., Lotz, J.M. & Shostak, W. (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal Parasitology, 83, 575–583. https://doi.org/10.2307/3284227
- Braga, M.P., Razzolini, E. & Boeger, W.A. (2015) Drivers of parasite sharing among Neotropical freshwater fishes. Journal of Animal Ecology, 84, 487–497. https://doi.org/10.1111/1365-2656.12298
- Cohen, S.C., Kohn, A. & Justo, M.C.N. (2013) South American Monogenoidea Parasites of Fishes, Amphibians and Reptiles. Oficina de Livros, Rio de Janeiro, 663 pp.
- Domingues, M.V. & Boeger, W.A. (2005) Neotropical Monogenoidea. 47. Phylogeny and coevolution of species of Rhinoxenus (Platyhelminthes, Monogenoidea, Dactylogyridae) and their Characiformes hosts (Teleostei, Ostariophysi) with description of four new species. Zoosystema, 27 (3), 441–467.
- Franceschini, L., Zago, A.C., Müller, M.I., Francisco, C.J., Takemoto, R.M. & Silva, R.J. (2017) Morphology and molecular characterization of Demidospermus spirophallus sp. n., D. prolixus sp. n. (Monogenea: Dactylogyridae) and a redescription of D. anus in siluriform catfish from Brazil. Journal of Helminthology, 92, 228–243. https://doi.org/10.1017/S0022149X17000256
- Franceschini, L., Acosta, A.A., Zago, A.A., Müller, M.I. & Da Silva, R.J. (2020) Trinigyrus spp. (Monogenea: Dactylogyridae) from Brazilian catfishes: new species, molecular data and new morphological contributions to the genus. Journal of Helminthology, 94 (e126), 1–15. https://doi.org/10.1017/S0022149X20000097
- Gibson, D. (2023) World List of Monogenea. In: Bánki, O., Roskov, Y., Döring, M., Ower, G., Hernández Robles, D.R., Plata Corredor, C.A., Stjernegaard Jeppesen, T., Örn, A., Vandepitte, L., Hobern, D., Schalk, P., DeWalt, R.E., Ma, K., Miller, J., Orrell, T., Aalbu, R., Abbott, J., Adlard, R., Adriaenssens, E.M., et al., Catalogue of Life Checklist. Version 12/2023. Available from: https://www.marinespecies.org (accessed 8 January 2024)
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321. https://doi.org/10.1093/sysbio/syq010
- Humason, G.L. (1979) Animal tissue techniques. W. H. Freemanco, Co., San Francisco, California, 661 pp.
- Jogunoori, W., Kritsky., D.C. & Venkatanarasaiah, J. (2004) Neotropical Monogenoidea. 46. Three new species from the gills of introduced aquarium fshes in India, the proposal of Heterotylus n. g. and Diaphorocleidus n g, and the reassignment of some previously described species of Urocleidoides Mizelle & Price, 1964 (Polyonchoinea: Dactylogyridae). Systematic Parasitology, 58, 115–124. https://doi.org/10.1023/B:SYPA.0000029422.16712.9a
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Mentjies, P. & Drummond, A. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Kritsky, D.C., Thatcher, V.E. & Boeger, W.A. (1988) Neotropical Monogenea. 13. Rhinosnastes pseudocapsaloideum n. gen., sp. n. (Dactylogyridae, Ancyrocephalinae), a nasal parasite of curimatã, Prochilodus nigricans Agassiz (Cypriniformes, Prochilodontidae), in Brazil. Journal of Parasitology, 74, 695–698. https://doi.org/10.2307/3282192
- Kritsky, D.C., Boeger, W.A. & Thatcher, V.E. (1988) Neotropical Monogenea.11. Rhinoxenus, new genus (Dactylogyridae, Ancyrocephalinae) with descriptions of three new species from the nasal cavities of Amazonian Characoidea. Proceedings of The Biological Society of Washington, 101, 87–94. [https://biostor.org/reference/74693]
- Kritsky, D.C., Van-Every, L.R. & Boeger, W.A. (1996) Neotropical Monogenoidea. 27. Two New Species of Telethecium n. gen. from the Nasal Cavities of Central Amazonian Fishes and a Redescription of Kritskyia moraveci Kohn, 1990 (Dactylogyridae, Ancyrocephalinae). Journal of Helminthology, 63 (1), 35–41.
- Kritsky, D.C. & Boeger, W.A. (1998) Neotropical Monogenoidea. 35. Pavanelliella pavanellii a New Genus and Species (Dactylogyridae, Ancyrocephalinae) from the Nasal Cavities of Siluriform Fishes in Brazil. Journal of Helminthology, 65 (2), 160–163.
- Kritsky, D.C. & Mendoza-Franco, E. (2003) Neotropical Monogenoidea. 42. Pavanelliella scaphiocotylus sp. nov. (Dactylogyridae) from the Nasal Cavity of the Guatemalan Chulin, Rhamdia guatemalensis (Siluriformes: Heptapteridae), from a Cenote on the Yucatán Peninsula, Mexico. Comparative Parasitology, 70 (2), 136–139. https://doi.org/10.1654/1525-2647(2003)070[0136:NMPSSN]2.0.CO;2
- Littlewood, D.T.J. & Olson, P.D. (2001) SSU rDNA and the Platyhelminthes: signal, noise, conflict and compromise. In: Littlewood, D.T.J. & Bray, R.A. (Eds.), Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp. 262–278.
- Mendoza‑Franco, E.F., Caspeta‑Mandujano, J.M. & Ramírez‑Martínez, C. (2019) Diaphorocleidus machacae n. sp. (Monogenea) Infecting the Gill Lamellae of Brycon guatemalensis (Characiformes: Bryconidae) from the Rio Lacantún Basin in Chiapas, Mexico. Acta Parasitologica, 64, 51–56. https://doi.org/10.2478/s11686-018-00007-7
- Mendoza-Palmero, C.A., Blasco-Costa, I. & Scholz, T. (2015) Molecular phylogeny of Neotropical monogeneans (Platyhelminthes: Monogenea) from catfishes (Siluriformes). Parasites & Vectors, 8, 1–11. https://doi.org/10.1186/s13071-015-0767-8
- Mendoza-Palmero, C.A., Mendoza-Franco, E.F., Acosta, A.A. & Scholz, T. (2019) Walteriella n. g. (Monogenoidea: Dactylogyridae) from the gills of pimelodid catfishes (Siluriformes: Pimelodidae) from the Peruvian Amazonia based on morphological and molecular data. Systematic Parasitology, 96, 441–452. https://doi.org/10.1007/s11230-019-09866-8
- Mizelle, J.D. & Price, C.E. (1963) Additional haptoral hooks in the genus Dactylogyrus. Journal of Parasitology, 49, 1028–1029. https://doi.org/10.2307/3275746
- Mizelle, J.D. & Klucka, A.R. (1953) Studies on Monogenetic Trematodes. XVI. Dactylogyridae from Wisconsin fishes. American Midland Naturalist, 49, 720–733. https://doi.org/10.2307/2485203
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. SC10 Workshop on Gateway Computing Environments (GCE10), New Orleans, Louisiana, 2010, 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Monteiro, C.M. & Brasil-Sato, M. (2014) A new species of Rhinonastes (Monogenoidea, Dactylogyridae), nasal parasite of Prochilodus argenteus (Actinopterygii, Characiformes) from Brazil. Acta Parasitologica, 59 (3), 540–543. https://doi.org/10.2478/s11686-014-0278-3
- Oliveira, G.S., da Silva, R.J., Vieira, F.E.G. & Acosta, A.A. (2021) Urocleidoides spp. (Monogenea: Dactylogyridae) from the gills of Parodon nasus (Characiformes: Parodontidae) from a Brazilian stream with descriptions of two new species. Zootaxa, 5081 (4), 535–550. https://doi.org/10.11646/zootaxa.5081.4.5
- Page, R.D.M. & Charleston, M.A. (1998) Trees within trees: phylogeny and historical associations. Tree, 13 (9), 356–359. https://doi.org/10.1016/S0169-5347(98)01438-4
- Posada, D. (2008) J ModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25, 1253–1256. https://doi.org/10.1093/molbev/msn083
- Rambaut, A. (2012) FigTree. Version 1.4. Molecular evolution, phylogenetics and epidemiology. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 30 June 2023)
- Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. (2014) Tracer. Version 1.6. World Wide Web electronic publication. Available from: http://tree.bio.ed.ac.uk/software/tracer/ (accessed 30 April 2023)
- Rasband, W.S. (2022) Imagej. US National Institutes of Health, Bethesda, Maryland. Available from: http://imagej.nih.gov/ij/ (accessed 30 June 2023)
- Reis, R.E., Albert, J.S., Di Dario, F., Mincarone, M.M., Petry, P. & Rocha, L.A. (2016) Fish biodiversity and conservation in South America. Journal of Fish Biology, 89 (1), 12–47. https://doi.org/10.1111/jfb.13016
- Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
- Rosim, D.F., Mendoza-Franco, E.F. & Luque, J.L. (2011) New and Previously Described Species of Urocleidoides (Monogenoidea: Dactylogyridae) Infecting the Gills and Nasal Cavities of Hoplias malabaricus (Characiformes: Erythrinidae) from Brazil. Journal of Parasitology, 97, 406–417. https://doi.org/10.1645/GE-2593.1
- Rossin, M.A., Francesco, P.N., Irigoitia, M.M., Scarabotti, P.A, Taglioretti, V. & Timi, J.T. (2019) Rhinoxenus (Dactylogyridae) parasitizing piranhas (Serrasalmidae) at its southernmost limit of distribution (Paraná River, Argentina), with the description of two new species. Anais da Academia Brasileira de Ciências, 91 (e2019071), 1–15. https://doi.org/10.1590/0001-3765201920190711
- Santos-Neto, J.F., Muriel-Cunha., J. & Domingues, M.V. (2019) New species of Anacanthorus (Dactylogyridae: Anacanthorinae) from the gills of Hoplerythrinus unitaeniatus and Erythrinus erythrinus (Characiformes: Erythrinidae) of the coastal drainage in the Eastern Amazon, Brazil. Zootaxa, 4615 (2), 303–320. https://doi.org/10.11646/zootaxa.4615.2.4
- Soares, G.B., Magalhães, K.X., Silva, A.C., Carneiro, J.S., Barbosa, L.L., Costa, N.G. & Domingues, M.V. (2019) Monogenoids (Polyonchoinea, Dactylogyridae) from Hydrolycus armatus (Characiformes, Cynodontidae) with the description of a new species of Rhinoxenus and the proposal of a new genus from the Xingu River, Pará, Brazil. Zootaxa, 4700 (2), 229–245. https://doi.org/10.11646/zootaxa.4700.2.3
- Wu, X.Y., Zhu, X.Q., Xie, M.Q. & Li, A.X. (2006) The radiation of Haliotrema (Monogenea: Dactylogyridae: Ancyrocephalinae): molecular evidence and explanation inferred from LSU rDNA sequences. Parasitology, 132, 659–668. https://doi.org/10.1017/S003118200500956X
- Zago, A.C., Yamada, F.H., Yamada, P.O.F., Franceschini, L., Bongiovani, M.F. & Da Silva, R.J. (2020) Seven new species of Urocleidoides (Monogenea: Dactylogyridae) from Brazilian fishes supported by morphological and molecular data. Parasitology Research, 119 (10), 3255–3283. https://doi.org/10.1007/s00436-020-06831-z
- Zago, A.C., Franceschini, L., Abdallah, V.D., Müller, M.I., Azevedo, R.K. & Silva, R.J. (2021) Morphological and molecular data of new species of Characithecium and Diaphorocleidus (Monogenea: Dactylogyridae) from Neotropical characid fishes. Parasitology International, 84, 102406. https://doi.org/10.1016/j.parint.2021.102406