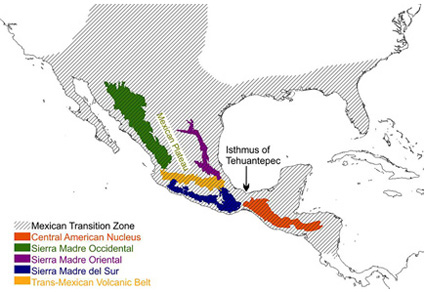
Abstract
Being areas of biotic overlap located between biogeographic regions, transition zones function as natural laboratories. The present study explores the phylogenetic history of the dung beetle subfamily Scarabaeinae, in order to present an evolutionary scenario that allows inference of the biogeographic history of the Mexican Transition Zone (MTZ) and integration of the distributional patterns of its biota. The species sampling included 94 New World taxa (93 species of Scarabaeinae and one species of Aphodiinae). The phylogenetic relationships of the main clades recovered in our study were supported with PP values ≥ 0.95. Based on the BAYAREALIKE model to reconstruct the ancestral distributional patterns of Scarabaeinae, we inferred a complex scenario with 19 dispersal events, 15 vicariance events, and three extinctions. We suggest that the Ancient Neotropical and Tropical Paleoamerican patterns represent the most likely ancestral distributional patterns for the Scarabaeinae of the MTZ, which probably settle there during the Eocene-Oligocene. The rest of the Scarabaeinae distributional patterns were assembled in subsequent periods. The results suggest that the MTZ had two separate formation stages: a Paleo-MTZ (Eocene-Miocene) and a current MTZ (Pliocene-Anthropocene). We conclude that the evolutionary history as well as the dispersal-vicariance scenario for the Scarabaeinae of the MTZ fits the “out of the tropics” model.
References
- Agnolin, F.L., Chimento, N.R. & Lucero, S.O. (2019) Pre-GABI biotic connections between the Americas: An alternative model to explain the “less-splendid isolation” of South America. Revista Geográfica de América Central, 61, 91–106.
- Ahrens, D., Schwarzer, J. & Vogler, A.P. (2014) The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proceedings of the Royal Society B: Biological Sciences, 281, 20141470. https://doi.org/10.1098/rspb.2014.1470
- Alfaro, M.E. & Huelsenbeck, J.P. (2006) Comparative performance of Bayesian and AIC-based measures of phylogenetic model uncertainty. Systematic Biology, 55, 89–96. https://doi.org/10.1080/10635150500433565
- Arillo, A. & Ortuño, V.M. (2008) Did dinosaurs have any relation with dung-beetles? (The origin of coprophagy). Journal of Natural History, 42, 1405–1408. https://doi.org/10.1080/00222930802105130
- Arita, H.T. & Vázquez-Domínguez, E. (2008) The tropics: cradle, museum or casino? A dynamic null model for latitudinal gradients of species diversity. Ecology Letters, 11, 653–663. https://doi.org/10.1111/j.1461-0248.2008.01197.x
- Bai, M., Li, S., Lu, Y., Yang, H., Tong, Y. & Yang, X. (2015) Mandible evolution in the Scarabaeinae (Coleoptera: Scarabaeidae) and adaptations to coprophagous habits. Frontiers in Zoology, 12, 30. https://doi.org/10.1186/s12983-015-0123-z
- Barnosky, A.D., Matzke, N., Tomiya, S., Wogan. G.O.U., Swartz, B., Quental, T.B., Marshall, C., McGuire, J.L., Lindsey, E.L., Maguire, K.C., Mersey, B. & Ferrer, E.A. (2011) Has the Earth’s sixth mass extinction already arrived? Nature, 471, 51–57. https://doi.org/10.1038/nature09678
- Barnosky, A.D., Lindsey, E.L., Villavicencio, N.A., Bostelmann, E., Hadly, E.A., Wanket, J. & Marshall, C.R. (2016) Variable impact of late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proceedings of the National Academy of Sciences, 113, 856–861. https://doi.org/10.1073/pnas.1505295112
- Barrier, E., Velasquillo, L., Chavez, M. & Gaulon, R. (1998) Neotectonic evolution of the Isthmus of Tehuantepec (southeastern Mexico). Tectonophysics, 287, 77–96. https://doi.org/10.1016/S0040-1951(98)80062-0
- Beebe, N.W. & Saul, A. (1995) Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction-restriction fragment length polymorphism analysis. American Journal of Tropical Medicine and Hygiene, 53, 478–481. https://doi.org/10.4269/ajtmh.1995.53.478
- Beynon, S.A., Wainwright, W.A. & Christie, M. (2015) The application of an ecosystem services framework to estimate the economic value of dung beetles to the U.K. cattle industry. Ecological Entomology, 40, 124–135. https://doi.org/10.1111/een.12240
- Breeschoten, T., Doorenweerd, C., Tarasov, S. & Vogler, A.P. (2016) Phylogenetics and biogeography of the dung beetle genus Onthophagus inferred from mitochondrial genomes. Molecular Phylogenetics and Evolution, 105, 86–95. https://doi.org/10.1016/j.ympev.2016.08.016
- Bromley, R.G., Buatois, L.A., Genise, J.F., Labandeira, C.G., Mángano, M.G., Melchor, R.N., Schlirf, M. & Uchman, A. (2007) Comments on the paper “Reconnaissance of Upper Jurassic Morrison Formation ichnofossils, Rocky Mountain Region, USA: paleoenvironmental, stratigraphic, and paleoclimatic significance of terrestrian and freshwater ichnocoenoses” by Stephen T.Hasiotis. Sedimentary Geology, 200, 141–150. https://doi.org/10.1016/j.sedgeo.2006.11.007
- Buchs, D.M., Irving, D., Coombs, H., Miranda, R., Wang, J., Coronado, M., Arrocha, R., Lacerda, M., Goff, C., Almengor, E., Portugal, E., Franceschi, P., Chichaco, E. & Redwood, S.D. (2019) Volcanic contribution to emergence of Central Panama in the Early Miocene. Scientific Reports, 9, 1417. https://doi.org/10.1038/s41598-018-37790-2
- Cano, E.B. (1998) Deltochilum valgum acropyge Bates (Coleoptera: Scarabaeidae: Scarabaeinae): habits and distribution. The Coleopterists Bulletin, 52, 174–178.
- Carvalho, M.R., Jaramillo, C., de la Parra, F., Caballero-Rodríguez, D., Herrera, F., Wing, S., Turner, B.L., D’Apolito, C., Romero-Báez, M., Narváez, P., Martínez, C., Gutierrez, M., Labandeira, C., Bayona, G., Rueda, M., Paez-Reyes, M., Cárdenas, D., Duque, Á., Crowley, J.L., Santos, C. & Silvestro, D. (2021) Extinction at the end-Cretaceous and the origin of modern Neotropical rainforests. Science, 372, 63–68. https://doi.org/10.1126/science.abf1969
- Chávez, R.P. (1993) Estudio palinológico de las floras fósiles del Mioceno inferior y principios del Mioceno medio de la región de Pichucalco, Chiapas, México. Acta Botánica Mexicana, 24, 1–96. https://doi.org/10.21829/abm24.1993.677
- Chin, K. & Gill, B.D. (1996) Dinosaurs, dung beetles, and conifers: participants in a Cretaceous food web. Palaios, 11, 280–285. https://doi.org/10.2307/3515235
- Cook, J. (1998) A revision of the Neotropical genus Bdelyrus Harold (Coleoptera: Scarabaeidae). The Canadian Entomologist, 130, 631–689. https://doi.org/10.4039/Ent130631-5
- Crews, S.C. & Esposito, L.A. (2020) Towards a synthesis of the Caribbean biogeography of terrestrial arthropods. BMC Evolutionary Biology, 20, 12. https://doi.org/10.1186/s12862-019-1576-z
- Cristóvão, J.P. & Lyal, C.H.C. (2018) Anchonini in Africa: New species and genus confirming a transatlantic distribution (Coleoptera: Curculionidae: Molytinae). Diversity, 10, 1–34. https://doi.org/10.3390/d10030082
- Cupello, M. (2022) Systematics of the enigmatic South American Streblopus Van Lansberge, 1874 dung beetles and their transatlantic origin: a case study on the role of dispersal events in the biogeographical history of the Scarabaeinae (Coleoptera: Scarabaeidae). European Journal of Taxonomy, 603, 1–85. https://doi.org/10.5852/ejt.2020.603
- Cupello, M. (2023) Mammalian islanders and dung beetles: a clarification. Scarabaeus, 3, 19–28.
- Cupello, M., Silva, F.A.B. & Vaz-de-Mello, F.Z. (2023) The Taxonomic Revolution of New World dung beetles (Coleoptera Scarabaeidae: Scarabaeinae). Frontiers in Ecology and Evolution, 11, 1168754. https://doi.org/10.3389/fevo.2023.1168754
- de Oliveira F.B., Molina E.C. & Marroig G. (2009) Paleogeography of the South Atlantic: A route for primates and rodents into the New World? In: Garber, P.A., Estrada, A., Bicca-Marques, J.C. & Heymann, E.W. (Eds.), South American Primates, Developments in Primatology: Progress and Prospects. Springer-Verlag, New York, New York, pp. 55–68. https://doi.org/10.1007/978-0-387-78705-3_3
- Davis, A.L.V., Scholtz, C.H. & Philips, T.K. (2002) Historical biogeography of scarabaeine dung beetles. Journal of Biogeography, 29, 1217–1256. https://doi.org/10.1046/j.1365-2699.2002.00776.x
- Davis, A.L.V. (2009) Section D. Historical biogeography of the Scarabaeinae and its physical and biotic drivers. In: Scholtz, C.H., Davis, A.L.V. & Kryger, U. (Eds.), Evolutionary biology and conservation of dung beetles. Pensoft, Sofia, pp. 329–385.
- Davis, A.L.V., Scholtz, C.H. & Sole, C.L. (2016) Biogeographical and co-evolutionary origins of scarabaeine dung beetles: Mesozoic vicariance versus Cenozoic dispersal and dinosaur versus mammal dung. Biological Journal of the Linnean Society, 120, 258–273. https://doi.org/10.1111/bij.12893
- Denk, T., Grímsson, F. & Zetter, R. (2010) Episodic migration of oaks to Iceland: Evidence for a North Atlantic “Land Bridge” in the latest Miocene. American Journal of Botany, 97, 276–287. https://doi.org/10.3732/ajb.0900195
- Dietz, L., Seidel, M., Eberle, J., Misof, B., Pacheco, T.L., Podsiadlowski, L., Ranisinghe, S., Gunter, N.L., Niehuis, O., Mayer, C. & Ahrens, D. (2023) A transcriptome-based phylogeny of Scarabaeoidea confirms the sister group relationship of dung beetles and phytophagous pleurostict scarabs (Coleoptera). Systematic Entomology, 48, 672–686. https://doi.org/10.1111/syen.12602
- Drummond, A.J., Suchard, M.A., Xie, D. & Rambaut, A. (2012) Bayesian phylogenetics with BEAUti and BEAST 1.7. Molecular Biology and Evolution, 29, 1969–1973. https://doi.org/10.1093/molbev/mss075
- Dugès, E. (1902) Algo sobre la distribución geográfica de algunas aves. Memorias de la Sociedad Científica Antonio Alzate, 18, 44–46.
- Ebert, K.M., Monteith, G.B., Menéndez, R. & Merritt, D.J. (2019) Bait preferences of Australian dung beetles (Coleoptera: Scarabaeidae) in tropical and subtropical Queensland forests. Austral Entomology, 58, 772–782. https://doi.org/10.1111/aen.12396
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340
- Edmonds, W.D. (1994) Revision of Phanaeus MacLeay, a New World genus of Scarabaeinae dung beetles. Contributions in Science of the Natural History Museum of Los Angeles County, 443, 1–105. https://doi.org/10.5962/p.208079
- Eguiluz de Antuñano, S., Marrett, R. & Aranda García, M. (2000) Tectónica de la Sierra Madre Oriental, México. Boletín de la Sociedad Geológica Mexicana, 53, 1–26. https://doi.org/10.18268/BSGM2000v53n1a1
- Faith, J.T. & Surovell, T.A. (2009) Synchronous extinction of North America’s Pleistocene mammals. Proceedings of the National Academy of Sciences, 106, 20641–20645. https://doi.org/10.1073/pnas.0908153106
- Favila, M.E. (2012) Historical, biogeographical and ecological factors explain the success of some native dung beetles after the introduction of cattle in Mexico. Pastos, 42, 161–181.
- Ferrari, L., Orozco-Esquivel, T., Manea, V. & Manea, M. (2012) The dynamic history of the Trans-Mexican Volcanic Belt and the Mexico subduction zone. Tectonophysics, 522–523, 122–149. https://doi.org/10.1016/j.tecto.2011.09.018
- Ferrari, L., Valencia-Moreno, M. & Bryan, S (2007) Magmatism and tectonics of the Sierra Madre Occidental and its relation with the evolution of the western margin of North America. Geological Society of America Special Papers, 422, 1–39. https://doi.org/10.1130/2007.2422(01)
- Ferro, I. & Morrone, J.J. (2014) Biogeographical transition zones: a search for conceptual synthesis. Biological Journal of the Linnean Society, 113, 1–12. https://doi.org/10.1111/bij.12333
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–9.
- Génier, F. (2015) Ateuchus cujuchi n. sp., a new inquiline species of Scarabaeinae (Coleoptera: Scarabaeidae) from tuco-tuco burrows in Bolivia. Zootaxa, 3946 (1), 146–148. https://doi.org/10.11646/zootaxa.3946.1.9
- Gillett, C.P.D.T. & Toussaint, E.F.A. (2020) Macroevolution and shifts in the feeding biology of the New World scarab beetle tribe Phanaeini (Coleoptera: Scarabaeidae: Scarabaeinae). Biological Journal of the Linnean Society, 130, 661–682. https://doi.org/10.1093/biolinnean/blaa058
- Giménez-Gómez, V.C, Verdú, J.R., Velazco, S.J.E. & Zurita, G.A. (2020) Dung beetle trophic ecology: are we misunderstanding resources attraction? Ecological Entomology, 46, 552–561. https://doi.org/10.1111/een.13001
- Graham, A. (1999) Studies in Neotropical Paleobotany. XIII. An Oligo-Miocene Palynoflora from Simojovel (Chiapas, México). American Journal of Botany, 86, 17–31. https://doi.org/10.2307/2656951
- Gou, P., Liu, Q., Xu, Y., Jiang, K., Hou, M., Ding, L., Pyron, R.A. & Burbrink, F.T. (2012) Out of Asia: Natricine snakes support the Cenozoic Beringian dispersal hypothesis. Molecular Phylogenetics and Evolution, 63, 825–833. https://doi.org/10.1016/j.ympev.2012.02.021
- Gunter, N.L., Weir, T.A., Slipinksi, A., Bocka, L. & Cameron, L. (2016) If dung beetles (Scarabaeidae: Scarabaeinae) arose in association with dinosaurs, did they also suffer a mass co-extinction at the K-Pg boundary? PLoS ONE, 11, e0153570. https://doi.org/10.1371/journal.pone.0153570
- Gunter, N.L., Monteith, G.B., Cameron, S.L. & Weir, T.A. (2018) Evidence from Australian mesic zone dung beetles supports their Gondwanan origin and Mesozoic diversification of the Scarabaeinae. Insect Systematics & Evolution, 50, 162–188. https://doi.org/10.1163/1876312X-00002171
- Gutiérrez-Velázquez, A., Rojas-Soto, O., Reyes-Castillo, P. & Halffter, G. (2012) The classic theory of Mexican Transition Zone revisited: the distributional congruence patterns of Passalidae (Coleoptera). Invertebrate Systematics, 27, 282–293. https://doi.org/10.1071/IS12056
- Hall, T.A. (1999) BioEdit: to user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Halffter, G. (1962) Explicación preliminar de la distribución geográfica de los Scarabaeidae mexicanos. Acta Zoológica Mexicana, Nueva Serie, 5, 1–17.
- Halffter, G. (1964) La entomofauna Americana, ideas acerca de su origen y distribución. Folia Entomológica Mexicana, 6, 1–108.
- Halffter, G. (1978) Un nuevo patrón de dispersión en la Zona de Transición Mexicana: el mesoamericano de montaña. Folia Entomológica Mexicana, 39–40, 219–222.
- Halffter, G. (1987) Biogeography of the montane entomofauna of Mexico and Central America. Annual Review of Entomology, 32, 95–114. https://doi.org/10.1146/annurev.en.32.010187.000523
- Halffter, G. (2017) La zona de transición mexicana y la megadiversidad de México: del marco histórico a la riqueza actual. Dugesiana, 24, 77–89.
- Halffter, G. (2019) La Zona de Transición Mexicana: Referente obligado para una nueva ponderación de la riqueza de México. In: Moreno, C.E. (Ed.), La biodiversidad en un mundo cambiante: Fundamentos Teóricos y metodológicos para su estudio. Universidad Autónoma del Estado de Hidalgo, Libermex, Pachuca de Soto, pp. 133–159.
- Halffter, G., Espinosa de los Monteros, A., Nolasco-Soto, J., Arriaga-Jiménez, A. & Rivera-Gasperín, S. (2022) Bajacanthon, a new subgenus for the Mexican Deltochilini (Coleoptera: Scarabaeidae: Scarabaeinae) fauna. Diversity, 14, 109. https://doi.org/10.3390/d14020109
- Halffter, G., Favila, M.E. & Arellano, L. (1995) Spatial distribution of three groups of Coleoptera along an altitudinal transect in the Mexican Transition Zone and its biogeographical implications. Elytron, 9, 151–185.
- Halffter, G. & Halffter, V. (1989) Behavioral evolution of the non-rolling roller beetles (Coleoptera: Scarabaeidae: Scarabaeinae. Acta Zoológica Mexicana, Nueva Serie, 32, 2–53. https://doi.org/10.21829/azm.1989.31321937
- Halffter, G. & Halffter, V. (2009) Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits or vegetable detritus. Boletín de la Sociedad Entomológica Aragonesa, 45, 1–22.
- Halffter, G. & Matthews, E.G. (1966) The natural history of dung beetles of the subfamily Scarabaeinae. Folia Entomológica Mexicana, 12–14, 1–321.
- Halffter, G. & Morrone, J.J. (2017) An analytical review of Halffter’s Mexican transition zone, and its relevance for evolutionary biogeography, ecology and biogeographical regionalization. Zootaxa, 4226 (1), 1–46. https://doi.org/10.11646/zootaxa.4226.1.1
- Halffter, G., Verdú, J.R., Márquez, J. & Moreno, C.E. (2008) Biogeographical analysis of Scarabaeinae and Geotrupinae along a transect in Central Mexico. Fragmenta Entomologica, 40, 273–322.
- Halffter, G., Zunino, M., Moctezuma, V. & Sánchez-Huerta, J.L. (2019) The integration processes of the distributional patterns in the Mexican Transition Zone: Phyletic, paleogeographic and ecological factors of a case study. Zootaxa, 4586 (1), 1–34. https://doi.org/10.11646/zootaxa.4586.1.1
- Heilprin, A. (1887) The geographical and geological distribution of animals. International Scientific Series, New York and London, 435 pp. https://doi.org/10.5962/bhl.title.28695
- Howden, H.F. & Cartwright, O.L. (1963) Scarab beetles of the genus Onthophagus Latreille north of Mexico (Coleoptera: Scarabaeidae). Proceedings of the United States National Museum, 114, 1–133. https://doi.org/10.5479/si.00963801.114-3467.1
- Hunter, T., Bergsten, J., Levkanicova, Z., Papadopoiulou, A., St. John, O., Wild, R., Hammond, P.M., Ahrens, D., Balke, M., Caterino, M.S., Gómez-Zurita, J., Ribera, I., Barraclough, T.G., Bocakova, M., Bocak, L. & Vogler, A.P. (2007) A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science, 318, 1913–1916. https://doi.org/10.1126/science.1146954
- Jablonski, D., Roy, K. & Valentine, J.W. (2006) Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science, 314, 102–106. https://doi.org/10.1126/science.1130880
- Jaramillo, C. (2018) Evolution of the Isthmus of Panama: Biological, Paleoceanographic and Paleoclimatological Implications. In: Hoorn, C., Perrigo, A. & Antonelli, A. (Eds.), Evolution of the Isthmus of Panama: Biological, Paleoceanographic and Paleoclimatological Implications. John Wiley and Sons Ltd., Hoboken, New Jersey, pp. 323–338.
- Jaramillo, C. (2019) 140 million years of tropical biome evolution. In: Gómez, J. & Pinilla-Pachon, A.O. (Eds.), The Geology of Colombia. Vol. 2. Mesozoic. Servicio Geológico Colombiano, Publicaciones Geológicas Especiales 36. Servicio Geológico Colombiano, Bogotá, pp. 1–28.
- Jeannel, R. (1942) La genèse des faunes terrestres: Éléments de biogéographie. Presses Universitaires de France, Paris, 513 pp.
- Kistler L., Montenegro Á., Smith B.D., Gifford J.A., Green R.E., Newsom L.A. & Shapiro B. (2014) Transoceanic drift and the domestication of African bottle gourds in the Americas. Proceedings of the National Academy of Sciences, 111, 2937‒2941. https://doi.org/10.1073/pnas.1318678111
- Kohlmann, B. & Solís, Á. (2001) El género Onthophagus (Coleoptera: Scarabaeidae) en Costa Rica. Giornale Italiano di Entomologia, 9, 159–261.
- Kohlmann, B., Solís, Á. & Alvarado, G.E. (2019) Description of Onthophagus humboldti and Uroxys bonplandi, two new scarab beetles (Coleoptera, Scarabaeidae, Scarabaeinae) from Costa Rica, with notes on tropical mountain brachyptery and endemicity. ZooKeys, 881, 23–51. https://doi.org/10.3897/zookeys.881.38026
- Kohlmann, B. & Vaz-de-Mello, F.Z. (2018) A new key for the species of Ateuchus Weber (Coleoptera: Scarabaeidae: Scarabaeinae) occurring in Mexico, with a description of the first North American inquiline species from a rodent burrow (Rodentia: Geomydae) and new distribution records. Revista Brasileira de Entomologia, 62, 131–134. https://doi.org/10.1016/j.rbe.2018.01.002
- Kohlmann-Cuesta, B.C. (2022) Escarabajos estercoleros (Aphodiidae, Geotrupidae y Scarabaeidae). In: CONABIO & SEMAEDESO (Eds.), La biodiversidad en Oaxaca. Estudio de estado. Vol. 3. CONABIO, Ciudad de México, pp. 61–72.
- Larsen, T.H., Lopera, A. & Forsyth, A. (2006) Extreme trophic habitat specialization by Peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 60, 315–324. https://doi.org/10.1649/0010-065X(2006)60[315:ETAHSB]2.0.CO;2
- Lavin, M. & Luckow, M. (1993) Origins and relationships of tropical North America in the context of the Boreotropics hypothesis. American Journal of Botany, 80, 1–14. https://doi.org/10.1002/j.1537-2197.1993.tb13761.x
- Lizardo, V., Moctezuma, V. & Escobar, F. (2022) Distribution, regionalization, and diversity of the dung beetle genus Phanaeus MacLeay (Coleoptera: Scarabaeidae) using species distribution models. Zootaxa, 5213 (5), 546–568. https://doi.org/10.11646/zootaxa.5213.5.4
- Lobo, J.M. & Halffter, G. (2000) Biogeographical and ecological factors affecting the altitudinal variation of mountainous communities of coprophagous beetles (Coleoptera: Scarabaeoidea): a comparative study. Annals of the Entomological Society of America, 93, 115–126. https://doi.org/10.1603/0013-8746(2000)093[0115:BAEFAT]2.0.CO;2
- Lopes, F., Gunter, N., Gillett, C.P.D.T., Montanaro, G., Rossini, M., Losacco, F., Daniel, G.M., Straube, N. & Tarasov, S. (2023a) From museum drawer to tree: historical DNA phylogenomics clarifies the systematics of rare dung beetles (Coleoptera: Scarabaeinae) from museum collections. BioRxiv. [published online] https://doi.org/10.1101/2023.10.27.564347
- Lopes, F., Rossini, M., Losacco, F., Montanaro, G., Gunter, N. & Tarasov, S. (2023b) Metagenomics reveals that dung beetles (Coleoptera: Scarabaeinae) broadly feed on reptile dung. Did they also feed on that of dinosaurs? Frontiers in Ecology and Evolution, 11, 1132729. https://doi.org/10.3389/fevo.2023.1132729
- Lumaret, J.P., Kadiri, N. & Martínez-M, I. (2020) The global decline of dung beetles. In: DellaSala, D.A. & Goldstein, M.I. (Eds.), Imperiled: The Encyclopedia of Conservation. Elsevier, Amsterdam, pp. 553–562. https://doi.org/10.1016/B978-0-12-821139-7.00018-0
- Maddison, W.P. & Maddison, D.R. (2010) Mesquite: a modular system for evolutionary analysis. Version 2.73. Available from: http://mesquiteproject.org (accessed 24 January 2024)
- Magalhaes, I.L.F., Martins, P.H., Faleiro, B.T., Vidigal, T.H.D.A., Santos, F.R., Carvalho, L.S. & Santos, A.J. (2023) Complete phylogeny of Micrathena spiders suggests multiple dispersal events among Neotropical rainforests, islands, and landmasses, and indicates Andean orogeny promotes speciation. BioRxiv. [published online] https://doi.org/10.1101/2023.11.01.565210
- Martínez-Hernández, E. & Ramírez-Arriaga, E. (1999) Palinoestratigrafía de la región de Tepeji de Rodríguez, Puebla, México. Implicaciones cronoestratigráficas. Revista Mexicana de Ciencias Geológicas, 16, 187–207.
- Manea, V.C. & Manea, M. (2006) The origin of modern Chiapanecan volcanic arc in southern Mexico inferred from thermal models. Special Paper of the Geological Society of America, 412, 27–38. https://doi.org/10.1130/2006.2412(02)
- Mastretta-Yanes, A., Moreno-Letelier, A., Piñero, D., Jorgensen, T.H. & Emerson, B.C. (2015) Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Trans-Mexican Volcanic Belt. Journal of Biogeography, 42, 1586–1600. https://doi.org/10.1111/jbi.12546
- Matschiner M. (2019) Gondwanan vicariance or trans-Atlantic dispersal of cichlid fishes: a review of the molecular evidence. Hydrobiologia, 832, 9–37. https://doi.org/10.1007/s10750-018-3686-9
- Matthews, E.G. (1961) A revision of the genus Copris Müller of the Western Hemisphere (Coleoptera, Scarabaeidae). Entomologica Americana, 41, 1–137.
- Matthews, E.G. (1966) A taxonomic and zoogeographic survey of the Scarabaeinae of the Antilles. (Coleoptera: Scarabaeidae). Memoirs of the American Entomological Society, 21, 111–134.
- Matthews, E.G. (1972) A revision of the Scarabaeinae dung beetles of Australia. I. Tribe Onthophagini. Australian Journal of Zoology, Supplementary Series, 9, 1–330. https://doi.org/10.1071/AJZS009
- Matthews, E.G. (1974) A revision of the Scarabaeinae dung beetles of Australia. II. Tribe Scarabaeini. Australian Journal of Zoology, Supplementary Series, 24, 1–211. https://doi.org/10.1071/AJZS038
- Matzke, N.J. (2013a) Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Frontiers of Biogeography, 5, 242–248. https://doi.org/10.21425/F5FBG19694
- Matzke, N.J. (2013b) BioGeoBEARS: BioGeography with Bayesian (and Likelihood) Evolutionary Analysis in R Scripts. Available from: http://CRAN.R-project.org/package=BioGeoBEARS (accessed 24 January 2024)
- Matzke, N.J. (2014) Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Systematic Biology, 63, 951–970. https://doi.org/10.1093/sysbio/syu056
- Mayr, G., Alvarenga, H. & Mourer-Chauviré, C. (2011) Out of Africa: Fossils shed light on the origin of the hoatzin, an iconic Neotropic bird. Naturwissenschaften, 98, 961–966. https://doi.org/10.1007/s00114-011-0849-1
- McKenna, D.D., Farrel, B.D., Caterino, M.S., Farnum, C.W., Hawks, D.C., Maddison, D.R., Seago, A.E., Short, A.E.Z., Newton, A.F. & Thayer, M.K. (2015) Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Systematic Entomology, 40, 35–60. https://doi.org/10.1111/syen.12093
- Miguez-Gutiérrez, A., Castillo, J., Márquez, J. & Goyenechea, I. (2013) Biogeografía de la Zona de Transición Mexicana con base en un análisis de árboles reconciliados. Revista Mexicana de Biodiversidad, 84, 215–224. https://doi.org/10.7550/rmb.32119
- Miller, S.E. (1983) Late Quaternary insects of Rancho La Brea and McKittrick, California. Quaternary Reseach, 20, 90–104. https://doi.org/10.1016/0033-5894(83)90067-4
- Miller, S.E., Gordon, R.D. & Howden, H.F. (1981) Reevaluation of Pleistocene scarab beetles from Rancho La Brea, California (Coleoptera: Scarabaeidae). Proceedings of the Entomological Society of Washington, 83, 625–630.
- Mlambo, S., Sole, C.L. & Scholtz, C.H. (2015) A molecular phylogeny of the African Scarabaeinae (Coleoptera: Scarabaeidae). Arthropod Systematics and Phylogeny, 73, 303–321. https://doi.org/10.3897/asp.73.e31806
- Moctezuma, V. & Halffter, G. (2019) New biogeographical makeup for colonisation of the Baja California Peninsula, with the description of a new Onthophagus (Coleoptera: Scarabaeidae: Scarabaeinae). Journal of Natural History, 53, 2057–2071. https://doi.org/10.1080/00222933.2019.1685694
- Moctezuma, V. & Halffter, G. (2021) Taxonomic revision of the Phanaeus endymion species group (Coleoptera: Scarabaeidae), with the descriptions of five new species. European Journal of Taxonomy, 747, 1–71. https://doi.org/10.5852/ejt.2021.747.1333
- Moctezuma, V., Halffter, G. & Escobar, F. (2016) Response of copronecrophagous beetle communities to habitat disturbance in two mountains of the Mexican Transition Zone: influence of historical and ecological factors. Journal of Insect Conservation, 20, 945–956. https://doi.org/10.1007/s10841-016-9923-5
- Moctezuma, V., Halffter, G. & Lizardo, V. (2021) The Phanaeus tridens species group (Coleoptera: Scarabaeoidea): a dung beetle group with genital morphological stasis but a changing ecological niche. Acta Entomologica Musei Nationalis Pragae, 61, 447–482. https://doi.org/10.37520/aemnp.2021.025
- Moctezuma, V., Halffter, G. & Mora-Aguilar, E.F. (2023) Una especie nueva del complejo de especies Onthophagus mirabilis (Coleoptera: Scarabaeidae: Scarabaeinae) de la región de Los Chimalapas, Oaxaca, México. Revista Mexicana de Biodiversidad, 94, e945081. https://doi.org/10.22201/ib.20078706e.2023.94.5081
- Moctezuma, V., Nogueira, G. & Halffter, G. (2020a) A revalidation and a new species in the genus Phanaeus (Coleoptera: Scarabaeoidea: Scarabaeidae: Scarabaeinae). Besoiro, 30, 3–11.
- Moctezuma, V., Sánchez-Huerta, J.L. & Halffter, G. (2018) Two new species of Ateuchus with remarks on ecology, distributions, and evolutionary relationships (Coleoptera, Scarabaeidae, Scarabaeinae). ZooKeys, 747, 71–86. https://doi.org/10.3897/zookeys.747.22731
- Moctezuma, V., Sánchez-Huerta, J.L. & Halffter, G. (2019) New species of Canthidium (Coleoptera: Scarabaeidae: Scarabaeinae) from Mexico. The Canadian Entomologist, 151, 432–441. https://doi.org/10.4039/tce.2019.25
- Moctezuma, V., Sánchez-Huerta, J.L. & Halffter, G. (2020b) Two new species of the Onthophagus clypeatus species group (Coleoptera: Scarabaeidae: Scarabaeinae). Florida Entomologist, 103, 281–287. https://doi.org/10.1653/024.103.0220
- Montes, C., Cardona, A., Jaramillo, C., Pardo, A., Silva, J.C., Valencia, V., Ayala, C., Pérez-Angel, L.C., Rodriguez-Parra, L.A., Ramirez, V. & Niño, H. (2015) Middle Miocene closure of the Central American Seaway. Science, 348, 226–229. https://doi.org/10.1126/science.aaa2815
- Morrone, J.J. (2004) Panbiogeografía, componentes bióticos y zonas de transición. Revista Brasileira de Entomologia, 48, 149–162. https://doi.org/10.1590/S0085-56262004000200001
- Morrone, J.J. (2014) Cladistic biogeography of the Neotropical region: identifying the main events in the diversification of the terrestrial biota. Cladistics, 30, 202–214. https://doi.org/10.1111/cla.12039
- Morrone, J.J. (2015a) Biogeographical regionalisation of the world: a reappraisal. Australian Systematic Botany, 28, 81–90. https://doi.org/10.1071/SB14042
- Morrone, J.J. (2015b) Halffter’s Mexican transition zone (1962–2014), cenocrons and evolutionary biogeography. Journal of Zoological Systematics and Evolutionary Research, 53, 249–257. https://doi.org/10.1111/jzs.12098
- Morrone, J.J. (2019) Regionalización biogeográfica y evolución biótica de México: encrucijada de la biodiversidad del Nuevo Mundo. Revista Mexicana Biodiversidad, 90, e902980. https://doi.org/10.22201/ib.20078706e.2019.90.2980
- Neita-Moreno, J.C., Agrain, F.A., Eberle, J., Ahrens, D. & Pereyra, V. (2019) On the phylogenetic position and systematics of extant and fossil Aclopinae (Coleoptera: Scarabaeidae). Systematic Entomology, 44, 709–727. https://doi.org/10.1111/syen.12366
- Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M.E. & Scarabaeinae Research Network (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation, 141, 1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
- Nieto-Samaniego, Á.F., Alaniz-Alvarez, S.A. & Camprubí, A. (2007) Mesa Central of Mexico: stratigraphy, structure, and Cenozoic tectonic evolution. Geological Society of America Special Papers, 422, 41–70. https://doi.org/10.1130/2007.2422(02)
- Nolasco-Soto, J., González-Astorga, J., Espinosa de los Monteros, A. & Favila, M.E. (2023) Evolutionary history and diversity in the ball roller beetle Canthon cyanellus. Frontiers in Ecology and Evolution, 10, 1066439. https://doi.org/10.3389/fevo.2022.1066439
- Nolasco-Soto, J., González-Astorga, J., Espinosa de los Monteros, A., Galante-Patiño, E. & Favila, M.E. (2017) Phylogeographic structure of Canthon cyanellus (Coleoptera: Scarabaeidae), a Neotropical dung beetle in the Mexican Transition Zone: Insights on its origin and the impacts of Pleistocene climatic fluctuations on population dynamics. Molecular Phylogenetics and Evolution, 109, 180–190. https://doi.org/10.1016/j.ympev.2017.01.004
- O’Dea, A., Lessios, H.A., Coates, A.G., Eytan, R.I., Restrepo-Moreno, S.A., Cione, A.L., Collins, L.S., de Queiroz, A., Farris, D.W., Norrisn, R.D., Stallard, R.F., Woodburne, M.O., Aguilera, O., Aubry, M.P., Berggren, W.A., Budd, A.F., Cozzuol, M.A., Coppard, S.E., Duque-Caro, H., Finnegan, S., Gasparini, G.M., Grossman, E.L., Johnson, K.G., Keigwin, L.D., Knowlton, N., Leigh, E.G., Leonard-Pingel, J.S., Marko, P.B., Pyenson, N.D., Rachello-Dolmen, P.G., Soibelzon, E., Soibelzon, L., Todd, J.A., Wermeij, G.J. & Jackson, J.B.C. (2016) Formation of the Isthmus of Panama. Science Advances, 2, e1600883. https://doi.org/10.1126/sciadv.1600883
- O’Leary, M.A., Bloch, J.I., Flynn, J.J., Gaudin, T.J., Giallombardo, A., Giannini, N.P., Goldberg, S.L., Kraatz, B.P., Luo, Z.X., Meng, J., Ni, X., Novacek, M.J., Perini, F.A., Randall, Z.S., Rougier, G.W., Sargis, E.J., Silcox, M.T., Simmons, N.B., Spaulding, M., Velazco, P.M., Weksler, M., Wible, J.R. & Cirranello, A.L. (2013) The Placental mammal ancestor and the post–K-Pg radiation of placentals. Science, 339, 662–667. https://doi.org/10.1126/science.1229237
- Oliveros, C.H., Andersen, M.J., Hosner, P.A., Mauck III, W.M., Sheldon, F.H., Cracraft, J. & Moyle, R.G. (2019) Rapid Laurasian diversification of a pantropical bird family during the Oligocene-Miocene transition. Ibis, 162, 137–152. https://doi.org/10.1111/ibi.12707
- Palacios-Chávez, R. & Rzedowski, J. (1993) Estudio palinológico de las floras fósiles del Mioceno inferior y principios del Mioceno Medio en la región de Pichucalco, Chiapas, México. Acta Botánica Mexicana, 24, 1–96. https://doi.org/10.21829/abm24.1993.677
- Posada, D. (2008) JModelTest: phylogenetic model averaging. Molecular Biology Evolution, 25, 1253–1256. https://doi.org/10.1093/molbev/msn083
- Poux, C., Chevret, P., Huchon, D., de Jong, W.W. & Douzery, E.J.P. (2006) Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Systematic Biology, 55, 228–244. https://doi.org/10.1080/10635150500481390
- Price, D. (2009) Phylogeny and biogeography of the dung beetle genus Phanaeus (Coleoptera: Scarabaeidae). Systematic Entomology, 34, 137–150. https://doi.org/10.1111/j.1365-3113.2008.00443.x
- Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. (2014) Tracer. Version 1.6. Available from: http://beast.bio.ed.ac.uk/Tracer (accessed 24 January 2024)
- Renner S. (2004) Plant dispersal across the tropical Atlantic by wind and sea currents. International Journal of Plant Sciences, 165, S23‒S33. https://doi.org/10.1086/383334
- Rodrigues-Aquino, P.S., Gonçalves de Jesus, F., Cândido Rocha, E., Castro Della Lucia, T.M., Cola Zanuncio, J. & da Silva Araújo, M. (2018) Predation rates of a beetle (Canthon virens) that kills female leaf-cutting ants (Atta spp.). International Journal of Agriculture and Biology, 20, 1247–1250.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Ruiz-Sánchez, E. & Ornelas, J.F. (2014) Phylogeography of Liquidambar styraciflua (Altingiaceae) in Mesoamerica: survivors of a Neogene widespread temperate forest (or cloud forest) in North America? Ecology and Evolution, 4, 311–328. https://doi.org/10.1002/ece3.938
- Sánchez-Huerta, J.L., Tonelli, M., Zunino, M. & Halffter, G. (2015) Redescription of Onthophagus halffteri Zunino (Coleoptera: Scarabaeidae: Scarabaeinae), with Ecological and distributional notes. The Coleopterists Bulletin, 69, 225–230. https://doi.org/10.1649/0010-065X-69.2.225
- Sánchez-Huerta, J.L., Zunino, M. & Halffter, G. (2018) A new species of American Onthophagus Latreille (Coleoptera: Scarabaeidae: Scarabaeinae) associated with rodent (Geomydae) burrows. The Coleopterists Bulletin, 72, 407–416. https://doi.org/10.1649/0010-065X-72.3.407
- Sanmartin, I., Enghoff, H. & Ronquist, F. (2001) Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biological Journal of the Linnean Society, 73, 345–390. https://doi.org/10.1006/bijl.2001.0542
- Schoolmeesters, P. (2023) World Scarabaeidae Database. Catalogue of Life. Naturalis, Leiden. Available from: https://www.checklistbank.org/dataset/272972/source/1027 (accessed 24 January 2024) https://doi.org/10.48580/ddz4x-38g
- Schwery, O. & O’Meara, B.C. (2021) Age, origin, and biogeography: unveiling the factors behind the diversification of dung beetles. BioRxiv. [published online] https://doi.org/10.1101/2021.01.26.428346
- Silvestro, D., Tejedor, M.F., Serrano-Serrano, M.L., Loiseau, O., Rossier, V., Rolland, J., Zizka, A., Höhna, S., Antonelli, A. & Salamin, N. (2019) Early arrival and climatically-linked geographic expansion of New World monkeys from tiny African ancestors. Systematic Biology, 68, 78‒92. https://doi.org/10.1093/sysbio/syy046
- Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H. & Flook, P. (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 87, 651–701. https://doi.org/10.1093/aesa/87.6.651
- Sole, C.L. & Scholtz, C.H. (2010) Did dung beetles arise in Africa? A phylogenetic hypothesis based on five gene regions. Molecular Phylogenetics and Evolution, 56, 631–641. https://doi.org/10.1016/j.ympev.2010.04.023
- Solórzano-Kraemer, M.M. (2007) Systematics, palaeoecology, and palaeobiogeography of the insect fauna from Mexican amber. Palaeontographica, Abteilung A, 282, 1–133. https://doi.org/10.1127/pala/282/2007/1
- Suárez-Mota, M.E., Téllez-Valdés, O., Lira-Saade, R. & Villaseñor, J.L. (2013) Una regionalización de la faja volcánica transmexicana con base en su riqueza florística. Botanical Sciences, 91, 93–105. https://doi.org/10.17129/botsci.405
- Takiya, D.M., Tran, P.L., Dietrich, C.H. & Moran, N.C. (2006) Co-cladogenesis spanning three phyla: leafhoppers (Insecta: Hemiptera: Cicadellidae) and their dual bacterial symbionts. Molecular Ecology, 15, 4175–4191. https://doi.org/10.1111/j.1365-294X.2006.03071.x
- Tarasov, S. & Dimitrov, D. (2016) Multigene phylogenetic analysis redefines dung beetles relationships and classification (Coleoptera: Scarabaeidae: Scarabaeinae). BMC Evolutionary Biology, 16, 257. https://doi.org/10.1186/s12862-016-0822-x
- Tarasov, S & Génier, F. (2015) Innovative Bayesian and Parsimony phylogeny of dung beetles (Coleoptera, Scarabaeidae, Scarabaeinae) enhanced by ontology-based partitioning of morphological characters. PLoS ONE, 10, e0116671. https://doi.org/10.1371/journal.pone.0116671
- Tarasov, S., Vaz-de-Mello, F.Z., Krell, F.T. & Dimitrov, D. (2016) A review and phylogeny of Scarabaeine dung beetle fossils (Coleoptera: Scarabaeidae: Scarabaeinae), with the description of two Canthochilum species from Dominican amber. PeerJ, 4, e1988. https://doi.org/10.7717/peerj.1988
- Tello, F., Rossini, M., Pino, M. & Verdú, J.R. (2021a) Nuevos registros fósiles de Onthophagus pilauco Tello, Verdú, Rossini y Zunino, 2021 (Coleoptera: Scarabaeidae: Scarabaeinae), revelan un patrón morfológico único entre los Onthophagus americanos. Revista Chilena de Entomología, 47, 935–949. https://doi.org/10.35249/rche.47.4.21.19
- Tello, F., Verdú, J.R., Rossini, M. & Zunino, M. (2021b) Onthophagus pilauco sp. nov. (Coleoptera, Scarabaeidae): evidence of beetle extinction in the Pleistocene-Holocene transition in Chilean Northern Patagonia. ZooKeys, 1043, 133–145. https://doi.org/10.3897/zookeys.1043.61706
- Upham, N.S., Esselstyn, J.A. & Jetz, W. (2019) Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biology, 17, e3000494. https://doi.org/10.1371/journal.pbio.3000494
- Vidal, N., Azvolinsky, A., Cruaud, C. & Hedges, S.B. (2008) Origin of tropical American burrowing reptiles by transatlantic rafting. Biology Letters, 4, 115–118. https://doi.org/10.1098/rsbl.2007.0531
- Vinther, J., Nicholls, R. & Kelly, D.A. (2021) A cloacal opening in a non-avian dinosaur. Current Biology, 31, R182–R183. https://doi.org/10.1016/j.cub.2020.12.039
- Voelker, G., Rohwer, S., Outlaw, D.C. & Bowie, R.C.K. (2008) Repeated trans-Atlantic dispersal catalyzed a global songbird radiation. Global Ecology and Biogeography, 18, 41–49. https://doi.org/10.1111/j.1466-8238.2008.00423.x
- Vršanský, P., van de Kamp, T., Azar, D., Prokin, A., Vidlička, L. & Vagovič, P. (2013) Cockroaches probably cleaned up after dinosaurs. PLoS One, 8, e80560. https://doi.org/10.1371/journal.pone.0080560
- Whiting, A.S., Sites, J.W. Jr., Pellegrino, K.C.M. & Rodrigues, M.T. (2006) Comparing alignment methods for inferring the history of the New World lizard genus Mabuya (Squamata: Scincidae). Molecular Phylogenetics and Evolution, 38, 719–730. https://doi.org/10.1016/j.ympev.2005.11.011
- Wolfe, J.A. (1978) A paleobotanical interpretation of Tertiary climates in the Northern Hemisphere. American Scientist, 66, 694–703.
- Young, O.P. (1981) The attraction of Neotropical Scarabaeinae (Coleoptera: Scarabaeidae) to reptile and amphibian fecal material. The Coleopterists Bulletin, 35, 345–348.
- Yu, Y., Blair, C. & He, X. (2020) RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Molecular Biology and Evolution, 37, 604–606. https://doi.org/10.1093/molbev/msz257
- Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292, 686–693. https://doi.org/10.1126/science.1059412
- Zanazzi, A., Kohn, M.J., MacFadden, B.J. & Terry Jr., D.O. (2007) Large temperature drop across the Eocene-Oligocene transition in central North America. Nature, 445, 639–642. https://doi.org/10.1038/nature05551
- Zunino, M. & Halffter, G. (1988) Análisis taxonómico, ecológico y biogeográfico de un grupo americano de Onthophagus (Coleoptera: Scarabaeidae). Museo Regionale di Scienze Naturali, Monografie, 9, 1–211.
- Zunino, M. & Halffter, G. (1997) Sobre Onthophagus Latreille, 1802 americanos (Coleoptera: Scarabaeidae: Scarabaeinae). Elytron, 11, 157–178.
- Zunino, M. & Halffter, G. (2007) The association of Onthophagus Latreille, 1802 beetles (Coleoptera: Scarabaeinae) with vertebrate burrows and caves. Elytron, 21, 17–55.