Abstract
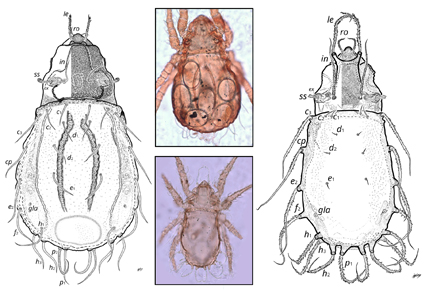
The oribatid mite genera Platynothrus and Heminothrus currently comprise 20 and 10 species, respectively, and collectively have a cosmopolitan distribution. They have been classified into three to five subgenera, depending on the classification. For Platynothrus, a couple of new species have been described in the last two years, while for Heminothrus, the last formal description of a new species was 26 years ago. In this study, we describe two new species of these crotoniid mites, discovered in the soil and litter of a tropical montane cloud forest in Mexico. One of these new species belongs to the genus Platynothrus, which can be distinguished from other species of this genus by the short clavate sensillus, curved smooth interlamellar seta, and the length of the notogastral setae. The second new species belongs to the genus Heminothrus, being characterized by a short rhomboidal sensillus and a very long interlamellar seta. Intraspecific genetic distances of the COX-1 mitochondrial marker were 2.15%, and 0.81% for the new Platynothrus and Heminothrus species, respectively.
References
- Barreto, C. & Lindo, Z. (2021) Checklist of oribatid mites (Acari: Oribatida) from two contrasting boreal fens: an update on oribatid mites of Canadian peatlands. Systematic & Applied Acarology, 26 (5), 866–884. https://doi.org/10.11158/saa.26.5.4
- Bast, J., Schaefer, I., Schwander, T., Maraun, M., Scheu, S. & Kraaijeveld, K. (2016) No accumulation of transposable elements in asexual arthropods. Molecular Biology and Evolution, 33, 697–706. https://doi.org/10.1093/molbev/msv261
- Behan-Pelletier, V. & Lindo, Z. (2023) Oribatid mites, Biodiversity, Taxonomy and Ecology. CRC Press, Boca Ratón, Florida, 508 pp. https://doi.org/10.1201/9781003214649
- Colloff, M.J. & Cameron, S.L. (2009) Revision of the oribatid mite genus Austronothrus Hammer (Acari: Oribatida): sexual dimorphism and a re-evaluation of the phylogenetic relationships of the family Crotoniidae. Invertebrate Systematics, 23, 87–110. https://doi.org/10.1071/IS08032
- van Dam, M.H., Trautwein, M., Spicer, G.S. & Esposito, L. (2018) Advancing mite phylogenomics: Designing ultraconserved elements for Acari phylogeny. Molecular Ecology Resources, 19, 465–475. https://doi.org/10.1111/1755-0998.12962
- Domes, K., Norton, R.A., Maraun, M. & Scheu, S. (2007) Reevolution of sexuality breaks Dollo´s law. Proceedings of the National Academy of Sciences, 104 (17), 7139–7144. https://doi.org/10.1073/pnas.0700034104
- Ermilov, S.G. & Khaustov, A.A. (2021) Some faunistical and taxonomic data on oribatid mites (Acari, Oribatida) from the vicinity of Lake Sivash (North Crimea). International Journal of Acarology, 47 (7), 603–609. https://doi.org/10.1080/01647954.2021.1971296
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3 (5), 294–299.
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Heethoff, M., Domes, K., Laumann, M., Maraun, M., Norton, R.A. & Scheu, S. (2007) High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). Journal of Evolutionary Biology, 20, 392–402. https://doi.org/10.1111/j.1420-9101.2006.01183.x
- Kammenga, J.E., van Gestel, C.A.M. & Hornung, E. (2001) Switching life-history sensitivities to stress in soil invertebrates. Ecological Applications, 11 (1), 226–238. https://doi.org/10.1890/1051-0761(2001)011[0226:SLHSTS]2.0.CO;2
- Kimura, M. (1980) A Simple Method for Estimating Evolutionary Rate of Base Substitutions through Comparative Studies of Nucleotide Sequences. Journal of Molecular Evolution, 16, 111–120. https://doi.org/10.1007/BF01731581
- Luo, W., Verweij, R.A. & van Gestel, C.A.M. (2015) Toxicity of Pb contaminated soils to the oribatid mite Platynothrus peltifer. Ecotoxicology, 24, 985–990. https://doi.org/10.1007/s10646-015-1439-3
- Melekhina, E.N. (2020) Oribatid mites as inhabitants of lichens in the taiga zone of northeastern Europe: biotopic association and ecological groups of species. Biology Bulletin, 47 (5), 522–534. https://doi.org/10.1134/S1062359020050064
- Öztoprak, H. & Bast, J. (2023) Modified salting out method for high molecular weight gDNA extraction (oribatid mites). protocols.io. [published online] https://doi.org/10.17504/protocols.io.yxmvm3yybl3p/v1
- Raspotnig, G., Krisper, G., Schuster, R., Fauler, G. & Leis, H.J. (2005) Volatile exudates from the oribatid mite Platynothrus peltifer. Journal of Chemical Ecology, 31 (2), 419–430. https://doi.org/10.1007/s10886-005-1350-0
- Raspotnig, G., Stabentheiner, E., Föttinger, P., Krisper, G. & Leis, H.J. (2008) Opisthonotal glands in the Camisiidae (Acari, Oribatida): evidence for a regressive evolutionary trend. Journal of Zoological Systematics and Evolutionary Research, 47 (1), 77–87. https://doi.org/10.1111/j.1439-0469.2008.00486.x
- Schatz, H., Behan-Pelletier, V.M., O’Connor, B.M. & Norton, R.A. (2011) Suborder Oribatida van der Hammen, 1968. In: Zhang, Z.-Q. (Ed.), Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148 (1), pp. 141–148. https://doi.org/10.11646/zootaxa.3148.1.26
- Seniczak, A., Seniczak, S., Hassel, K. & Ivar-Flatberg, K. (2022) Morphological ontogeny of Platynothrus troendelagicus sp. nov. (Acari, Oribatida, Camisiidae) from Norway. Systematic & Applied Acarology, 27 (9), 1702–1722. https://doi.org/10.11158/saa.27.9.2
- Siepel, H. (1990) Niche relationships between two panphytophagous soil mites, Nothrus silvestris Nicolet (Acari, Oribatida, Nothridae) and Platynothrus peltifer (Koch) (Acari, Oribatida, Camisiidae). Biology and Fertility of Soils, 9, 139–144. https://doi.org/10.1007/BF00335797
- Subías, L.S. (2022) Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (Excepto fósiles). (18ª actualización). Graellsia, 60, 3–305. [updated February 2023, http://bba.bioucm.es/cont/docs/RO_1.pdf] https://doi.org/10.3989/graellsia.2004.v60.iExtra.218
- Tamura, K., Stecher, G. & Kumar, S. (2021) MEGA11: Molecular Evolutionary Genetics Analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. https://doi.org/10.1093/molbev/msab120
- Weigmann, G. (2006) Hornmilben (Oribatida). Die Tierwelt Deutschlands. Vol. 76. Goecke & Evers, Keltern, 520 pp.