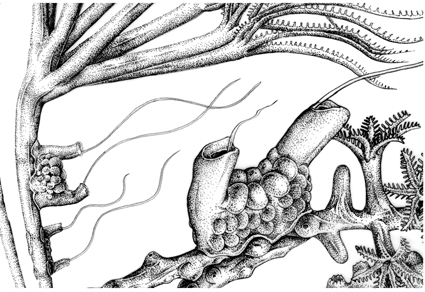
Abstract
Some of the most fascinating and poorly known animals on this planet are comb jellies of the phylum Ctenophora. About one-quarter of ctenophore richness is encompassed by the benthic species of the order Platyctenida, nearly all known from shallow waters. In this work, we integrate several systematic methods to elucidate an enigmatic genus, Tjalfiella, known previously only from deep waters near the western coastline of Greenland in the North Atlantic. For the first time, we employ microCT on museum specimens—one nearly 100 years old from the type locality of the only known species of the genus, T. tristoma—of extant ctenophores to visualize and compare their anatomy. With these data, we integrate in situ videography and genetic sequence data derived from newly collected deep sea specimens observed via NOAA Ship Okeanos Explorer in 2018 and 2022 at two distant localities in the North Atlantic, near North Carolina, USA, and the Azores, Portugal. The genetic data indicate that the newly collected specimens represent closely related but distinct species of Tjalfiella. However, neither can be named at this time because neither one could be definitively differentiated from T. tristoma, given that microCT and in situ imagery reveal striking morphological similarities and only variation in color and host preference. Despite the lack of new species descriptions, this work characterizes both the morphology and genetics of the benthic ctenophore genus Tjalfiella and specimens representing species within it, advancing our understanding of a rarely observed component of the deep-sea fauna.
References
- Allio, R., Schomaker-Bastos, A., Romiguier, J., Prosdocimi, F., Nabholz, B. & Delsuc, F. (2020) MitoFinder: Efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Molecular ecology resources, 20 (4), 892–905. https://doi.org/10.1111/1755-0998.13160
- Arafat, H., Alamaru, A., Gissi, C. & Huchon, D. (2018) Extensive mitochondrial gene rearrangements in Ctenophora: insights from benthic Platyctenida. BMC Evolutionary Biology, 18 (1), 65. https://doi.org/10.1186/s12862-018-1186-1
- Beazley, L., Kenchington, E., Murillo, F.J., Brickman, D., Wang, Z., Davies, A.J., Roberts, E.M. & Rapp, H.T. (2021) Climate change winner in the deep sea? Predicting the impacts of climate change on the distribution of the glass sponge Vazella pourtalesi. Marine Ecology Progress Series, 657, 1–23. https://doi.org/10.3354/meps13566
- Christianson, L. M., Johnson, S. B., Schultz, D. T. & Haddock, S. H. D. (2022) Hidden diversity of Ctenophora revealed by new mitochondrial COI primers and sequences. Molecular Ecology Resources, 22 (1), 283–294. https://doi.org/10.1111/1755-0998.13459
- Claudet, J., Amon, D.J. & Blasiak, R. (2021) Transformational opportunities for an equitable ocean commons. Proceedings of the National Academy of Sciences USA, 118 (42), e2117033118. https://doi.org/10.1073/pnas.2117033118
- Costello, M.J., Coll, M., Danovaro, R., Halpin, P., Ojaveer, H. & Miloslavich, P. (2010) A Census of Marine Biodiversity Knowledge, Resources, and Future Challenges. PLoS ONE, 5 (8), e12110. https://doi.org/10.1371/journal.pone.0012110
- Dawydoff, C. (1950) La nouvelle forme de Ctenophores planarises sessiles provenant de la Mer de Chine Meridionale (Savangia atentaculata nov. gen. nov. spec.). Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences, 231 (17), 814–816.
- Devanesen, D. & Varadarajan, S. (1942) On three new species of Coeloplana found at Krusadai Island, Marine Biological Station, and Gulf of Mannar. Journal of Madras University, 14 (2), 181–188.
- Dragonfly (2020) Dragonfly. Computer Software. Object Research Systems (ORS) Inc., Montreal. Available from: http://www.theobjects.com/dragonfly (accessed 24 July 2024)
- Dunn, C.W., Leys, S.P. & Haddock, S.H.D. (2015) The hidden biology of sponges and ctenophores. Trends in Ecology & Evolution, 30 (5), 282–291. https://doi.org/10.1016/j.tree.2015.03.003
- Eeckhaut, I., Flammang, P., Lo Bue, C. & Jangoux, M. (1997) Functional morphology of the tentacles and tentilla of Coeloplana bannworthi (Ctenophora, Platyctenida), an ectosymbiont of Diadema setosum (Echinodermata, Echinoida). Zoomorphology, 117 (3), 165–174. https://doi.org/10.1007/s004350050041
- Galvez, K., Cantwell, K., Suhre, K., Albano, P., Hoy, S., Rabenold, C., Cromwell, M., Ruby, C., Lienesch, A., France, S., Ganguly, U., Adams, C., Candio, S., Dornback, M., Wilkins, C., Maxon, A., Sorset, S., Copeland, A., Dunn, C., Gregory, T., Ritter, C., O’Brien, A., Gottfried, S., Howard, A., Brian, R., Kennedy, R.C., Lobecker, E., Guan, S., Ford, F., Ryan, M. & Medley, R. (2024) NOAA Ocean Exploration ROV and Telepresence Deepwater Exploration Procedures Manual. NOAA Ocean Exploration, National Oceanic and Atmospheric Administration, Silver Spring, Maryland. [unknown pagination]
- Glynn, P.W., Bayer, F.M. & Renegar, D.A. (2014) Coeloplana waltoni, a new species of minute benthic ctenophore (Ctenophora: Platyctenida) from south Florida. Proceedings of the Biological Society of Washington, 127 (2), 423. https://doi.org/10.2988/0006-324X127.2.423
- Glynn, P.W., Coffman, B., Primov, K., Renegar, D.A., Gross, J., Blackwelder, P., Martinez, N., Dominguez, J., Vanderwoude, J. & Riegl, B.M. (2019) Benthic ctenophore (Order Platyctenida) reproduction, recruitment, and seasonality in south Florida. Invertebrate Biology, 138 (3), e12256. https://doi.org/10.1111/ivb.12256
- Good, E., Holman, L.E., Pusceddu, A., Russo, T., Rius, M. & Iacono, C.L. (2022) Detection of community-wide impacts of bottom trawl fishing on deep-sea assemblages using environmental DNA metabarcoding. Marine pollution bulletin, 183, 114062. https://doi.org/10.1016/j.marpolbul.2022.114062
- Guindon, S., Dufayard, J.-F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59 (3), 307–321. https://doi.org/10.1093/sysbio/syq010
- Harbison, G.R. (1985) On the classification and evolution of the Ctenophora. In: Conway Morris, S., George, G.D., Gibson, R. & Platt, H.M. (Eds.), The origin and relationships of lower invertebrates. Clarendon Press, Oxford, pp. 78–100.
- Harbison, G.R. & Volovik, S.P. (1994) The ctenophore, Mnemiopsis leidyi, in the Black Sea: a holoplanktonic organism transported in the ballast water of ships. Nonindigenous Estuarine and Marine Organisms, (NEMO), 25–36.
- Hillis, D.M. & Bull, J.J. (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology, 42 (2), 182–192. https://doi.org/10.1093/sysbio/42.2.182
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular biology and evolution, 30 (4), 772–780. https://doi.org/10.1093/molbev/mst010
- Komai, T. (1922) Studies on two aberrant ctenophores, Coeloplana and Gastrodes. Niodo Publishers, Kyoto, 102 pp., 9 pls. [20 June 1922] https://doi.org/10.5962/bhl.title.7006
- Komai, T. (1941) 49. A New Remarkable Sessile Ctenophore. Proceedings of the Imperial Academy, Tokyo, 17 (6), 216–220. https://doi.org/10.2183/pjab1912.17.216
- Korotneff, A. (1886) Ctenoplana kowalevskii. Zeitschrift für Wissenschaftliche Zoologie, 43, 242–251
- Kowalevsky, A. (1880) Coeloplana metschnikowii. Zoologischer Anzeiger, 3 (51), 140.
- Kramp, P.L. (1942) Ctenophora. In: The Godthaab Expedition 1928. Bianco Lunos Bogtrykkeri A/S. 80 (9). C. A. Reitzels Forlag, Kobenhavn, pp. 1–19.
- Lang, A. (1884) Die polycladen (seeplanarien) des golfes von Neapel und der angrenzenden meeres-abschnitte. W. Engelmann, Leipzig, 688 pp., 39 pls. https://doi.org/10.5962/bhl.title.10545
- Lefort, V., Longueville, J. & Gascuel, O. (2017) SMS: Smart Model Selection in PhyML. Molecular Biology and Evolution, 34 (9), 2422–2424. https://doi.org/10.1093/molbev/msx149
- Lusty, P.A.J. & Murton, B.J. (2018) Deep-ocean mineral deposits: Metal resources and windows into earth processes. Elements, 14, 301–306. https://doi.org/10.2138/gselements.14.5.301
- Mackie, G.O., Mills, C.E. & Singla, C.L. (1988) Structure and function of the prehensile tentilla of Euplokamis (Ctenophora, Cydippida). Zoomorphology, 107 (6), 319–337. https://doi.org/10.1007/BF00312216
- Metscher, B.D. (2009) MicroCT for comparative morphology: simple staining methods allow high contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology, 9, 11. https://doi.org/10.1186/1472-6793-9-11
- Mills, C.E. (2024) Internet (1998–present). Phylum Ctenophora: list of all valid species names. Electronic internet document. Ctenophora. Accessed through: World Register of Marine Species. Available from: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1248 (accessed 2 June 2024)
- Mortensen, T. (1910) Tjalfiella tristoma n. g., n. sp. A sessile ctenophore from Greenland. Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjøbenhavn, 17, 249–253.
- Mortensen, T. (1912) Papers from Dr. Th. Mortensen’s Pacific Expedition—The Danish Ingolf-Expedition: Ctenophora. Vol. 5. Part 2. H. Hagerup, Copenhagen, 59 pp., 15 text figs., 10 pls. https://doi.org/10.5962/bhl.title.23582
- Parker, G.H. (1905) The movements of the swimming plates in ctenophores, with reference to the theories of ciliary metachronism. Journal of Experimental Zoology, 2 (3), 407–423. https://doi.org/10.1002/jez.1400020306
- Phillips, A.J. & Goetz, F.E. (2023) Comparative reproductive morphology of two species of Macrobdella (Hirudinea: Arhynchobdellida: Macrobdellidae). Zoomorphology, 142 (2), 153–168. https://doi.org/10.1007/s00435-023-00596-6
- Podar, M., Haddock, S.H.D., Sogin, M.L. & Harbison, G.R. (2001) A Molecular Phylogenetic Framework for the Phylum Ctenophora Using 18S rRNA Genes. Molecular Phylogenetics and Evolution, 21 (2), 218–230. https://doi.org/10.1006/mpev.2001.1036
- Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. 2020. Using SPAdes De Novo Assembler. Current protocols in bioinformatics, 70 (1), e102. https://doi.org/10.1002/cpbi.102
- Rabone, M., Wiethase, J.H., Simon-Lledó, E., Emery, A.M., Jones, D.O.B., Dahlgren, T.G., Bribiesca-Contreras, G., Wiklund, H., Horton, T. & Glover, A.G. (2023) How many metazoan species live in the world‘s largest mineral exploration region? Current biology, 33 (12), e5, 2383–2396. https://doi.org/10.1016/j.cub.2023.04.052
- Rankin, J.J. (1956) The structure and biology of Vallicula multiformis, gen. et sp. nov., a platyctenid ctenophore. Journal of the Linnean Society of London, Zoology, 43 (289), 55–71. https://doi.org/10.1111/j.1096-3642.1956.tb02507.x
- Robilliard, G.A. & Dayton, P.K. (1972). A new species of platyctenean ctenophore, Lyrocteis flavopallidus sp. nov., from McMurdo Sound, Antarctica. Canadian Journal of Zoology, 50 (1), 47–52. https://doi.org/10.1139/z72-009
- Shifu, C., Yanqing, Z., Yaru, C. & Jia, G. (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics, 34 (17), i884–i890. https://doi.org/10.1093/bioinformatics/bty560
- Simion, P., Bekkouche, N., Jager, M., Quéinnec, E. & Manuel, M. (2015) Exploring the potential of small RNA subunit and ITS sequences for resolving phylogenetic relationships within the phylum Ctenophora. Zoology, 118 (2), 102–114. https://doi.org/10.1016/j.zool.2014.06.004
- Tamm, S. & Tamm, S.L. (1988) Development of macrociliary cells in Beroë. II. Formation of macrocilia. Journal of Cell Science, 89, 81–95. https://doi.org/10.1242/jcs.89.1.81
- Thiel, H. (1968) Coeloplana meteoris nov. spec.(Ctenophora: Platyctenea): Bescheibung und systematische Stellung mit einem Vergleich der Gastrovascularsysteme in deiser Ordung. Meteor Forschungsergebnisse, Series D, 3, 1–13.
- Townsend, J.P., Tassia, M.G. & Damian-Serrano, A. (2020) A mesopelagic ctenophore representing a new family, with notes on family-level taxonomy in Ctenophora: Vampyroctena delmarvensis gen. nov. sp. nov. (Vampyroctenidae, fam. nov.). Marine Biodiversity, 50, 34. https://doi.org/10.1007/s12526-020-01049-9
- Whelan, N.V., Kocot, K.M., Moroz, T.P., Mukherjee, K., Williams, P., Paulay, G., Moroz, L.L. & Halanych, K.M. (2017) Ctenophore relationships and their placement as the sister group to all other animals. Nature Ecology & Evolution, 1 (11), 1737–1746. https://doi.org/10.1038/s41559-017-0331-3
- Wiesenburg, D.A., Shipp, B., Fodrie, F.J., Powers, S., Lartigue, J., Darnell, K.M., Baustian, M.M., Ngo, C., Valentine, J.F. & Wowk, K. (2021) Prospects for Gulf of Mexico environmental recovery and restoration. Oceanography, 34 (1), 164–173. https://doi.org/10.5670/oceanog.2021.124
- Willey, A. (1896) On Ctenoplana. Journal of Cell Science, 2 (155), 323–342. https://doi.org/10.1242/jcs.s2-39.155.323
- Zhang, G., Feng, C., Yao, X., Ji, M., Yang, H., Qu, H., Zeng, Q., Zhao, Z. & Sun, R. (2021) Petroleum Geology in Deepwater Settings in a Passive Continental Margin of a Marginal Sea: A Case Study from the South China Sea. Acta Geologica Sinica, English Edition, 95 (1), 1–20. https://doi.org/10.1111/1755-6724.14621