Abstract
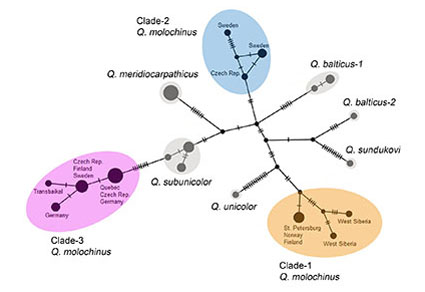
Phylogenetic assessment of COI barcodes from 22 specimens identified as Q. molochinus based on external morphology and shape of the aedeagus revealed three non-sister clades within this recently revised species, with large molecular distance (6.3–7.8%) among them, suggesting their species status. On the contrary, preliminary study of the aedeagal internal sac (endophallus) of the available males from these clades and from the more numerous additional non-sequenced materials of Q. molochinus did not reveal notable variants. We report this case here because firstly, this goes against some cases observed in other beetles where endophallic characters may be the only morphological traits supporting molecular-based cryptic species, and secondly, these molecular clades are unexpected within a species we thought to be well-known. DNA barcoding, exploration of nuclear DNA markers and an in-depth examination of the fully everted endophallus for a wider sample of freshly collected specimens are required for further study and explanation of the detected molecular polymorphism of Q. molochinus. An illustration of the everted internal sac as a reference and new distributional data for this species are provided.
References
- Brunke, A.J., Salnitska, M., Hansen, A.K., Zmudzinska, A., Smetana, A., Buffam, J & Solodovnikov, A. (2020a) Are subcortical rove beetles truly Holarctic? An integrative taxonomic revision of north temperate Quedionuchus (Coleoptera: Staphylinidae: Staphylininae). Organisms Diversity & Evolution, 20, 77–116. https://doi.org/10.1007/s13127-019-00422-2
- Clement, M., Snell, Q., Walker, P., Posada, D. & Crandall, K. (2002) TCS: estimating gene genealogies. [Paper presentation]. Parallel and Distributed Processing Symposium, International, April). Vol. 3. IEEE Computer Society, (Vol. 3, pp. 0184. Available from: https://ieeexplore.ieee.org/document/1016585 (accessed 23 September 2024)–0184). https://doi.org/10.1109/IPDPS.2002.1016585
- Dyer, K.A., Burke, C. & Jaenike, J. (2011) Wolbachia-mediated of mtDNA from a potentially extinct species. Molecular Ecology, 20, 2805-2817. https://doi.org/10.1111/j.1365-294X.2011.05128.x
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
- Fossen, E.I., Ekrem, T., Nilsson, A.N. & Bergsten, J. (2016) Species delimitation in northern European water scavenger beetles of the genus Hydrobius (Coleoptera, Hydrophilidae). ZooKeys, 564, 71–120. https://doi.org/10.3897/zookeys.564.6558
- Gebremeskel, A., Salnitska, M., Krivosheeva, V. & Solodovnikov, A. (2023) Micro-endemism pattern and Wolbachia infection of Quedius obliqueseriatus (Coleoptera, Staphylinidae), a montane rove beetle endemic of the North-Western Caucasus. Alpine Entomology, 7, 153–166. https://doi.org/10.3897/alpento.7.111214
- Geisler, L., Hansen, A.K., Shaw, J.J. & Solodovnikov, A. (2024) Integrative taxonomy suggests species status for Philonthus sideropterus Kolenati, 1846 sp. propr., the former color form of Philonthus splendens (Fabricius, 1793) (Coleoptera: Staphylinidae). Integrative Systematics, 7 (1), 9–23. https://doi.org/10.18476/2024.210366
- Goreslavets, I.N. (2016) First addition to the rove beetle (Coleoptera, Staphylinidae) fauna and ecology of Krasnosamarsky forest. Samara Luka: problems of regional and global ecology, 25 (4), 115–122. [in Russian]
- Gravenhorst, J.L.C. (1806) Monographia Coleopterorum Micropterorum. H. Dieterich, Gottingae, 248 pp. https://doi.org/10.5962/bhl.title.67769
- Guindon, S. & Gascuel, O. (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology, 52, 696–704. https://doi.org/10.1080/10635150390235520
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Hansen, A.K., Brunke, A., Simonsen, T. & Solodovnikov, A. (2022) Revision of Quedius sensu stricto (Coleoptera: Staphylinidae). Acta Entomologica, 62 (1), 225–299. https://doi.org/10.37520/aemnp.2022.017
- Hebert, P.D., Cywinska, A. & Ball, S.L. (2003) Biological identifications through DNA barcodes. Proceedings of the Royal Society B, Biological Sciences, 270, 313–321. https://doi.org/10.1098/rspb.2002.2218
- Hebert, P.D.N., Bock, D.G. & Prosser, S.W.J. (2023) Interrogating 1000 insect genomes for NUMTs: A risk assessment for estimates of species richness. PLoS ONE, 18 (6), e0286620. https://doi.org/10.1371/journal.pone.0286620
- Ivanov, V., Lee, K.M. & Mutanen, M. (2018) Mitonuclear discordance in wolf spiders: Genomic evidence for species integrity and introgression. Molecular Ecology, 27, 1681–1695. https://doi.org/10.1111/mec.14564
- Jordal, B.H. & Kambestad, M. (2014) DNA barcoding of bark and ambrosia beetles reveals excessive NUMTs and consistent east-west divergence across Palearctic forests. Molecular Ecology Resources, 14, 7–17. https://doi.org/10.1111/1755-0998.12150
- Kiesenwetter, H. Von & Märkel, F. (1847) Eine entomologische Excursion im Riesengebirge im Juli 1846 (Fortsetzung). Entomologische Zeitung, Stettin, 8, 73–78.
- Korge, H. (1960) Eine neue deutsche Quedius-Art von der Ostseeküste (Col. Staphylinidae). Mitteilungen der Deutschen Entomologischen Gesellschaft, 19, 52–53. https://doi.org/10.1002/mmnd.4820190306
- Korge, H. (1961) Quedius subunicolor n. sp. aus Nordeuropa (Col. Staphyl.). Mitteilungen der Deutschen Entomologischen Gesellschaft, 20, 81–83.
- Lee, J., Lee, J.S. & Ahn, K.J. (2020) Reappraisal of Korean Oxyporus Fabricius (Coleoptera: Staphylinidae: Oxyporinae), including a new species based on morphological and molecular characters. Journal of Asia-Pacific Entomology, 23 (3), 680–688. https://doi.org/10.1016/j.aspen.2020.04.012
- Leigh, J.W. & Bryant, D. (2015) POPART: Full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6 (9), 1110–1116. https://doi.org/10.1111/2041-210X.12410
- Majka, C.G. (2007) Quedius molochinus (Coleoptera: Staphylinidae) newly recorded in the Maritime Provinces of Canada. Proceedings of the Entomological Society of Washington, 109 (4), 958–959.
- Ménétriés, E. (1832) Catalogue raisonné des objets de zoologie recueillis dans un voyage au Caucase et jusqu’aux frontières actuelles de la Perse entrepris par ordre de S. M. L’Empereur. L’Académie Impériale des Sciences, St. Petersbourg, 271 + xxxii + iv pp. https://doi.org/10.5962/bhl.title.51784
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. [Paper presentation]. Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, November, 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Muñoz-Tobar, S.I & Caterino, M.S. (2020) Mountains as Islands: Species Delimitation and Evolutionary History of the Ant-Loving Beetle Genus Panabachia (Coleoptera, Staphylinidae) from the Northern Andes. Insects, 11, 64. https://doi.org/10.3390/insects11010064
- Osella, G. & Zanetti, A. (1975) La coleotterofauna dei nidi di Talpa europaea L. nell’Italia settentrionale a nord del fiume Po. Bollettino di Zoologia agraria e di Bachicoltura, Series 2, 12 (1974), 41–200.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Runde, G.H. (1835) Brachelytrorum species Agri Halensis. Ploetzianis, Halae, pp. 32.
- Salnitska, M. & Solodovnikov, A. (2019) Rove beetles of the genus Quedius (Coleoptera, Staphylinidae) of Russia: a key to species and annotated catalogue. ZooKeys, 847, 1–100. https://doi.org/10.3897/zookeys.847.34049
- Salnitska, M. & Solodovnikov, A. (2021) DNA barcode sheds light on species boundaries in the common morphologically variable rove beetle Quedius umbrinus-complex that puzzled taxonomists for more than a century (Coleoptera, Staphylinidae). Systematics and Biodiversity, 19, 859–874. https://doi.org/10.1080/14772000.2021.1943559
- Salokannel, J., Lee, K.M., Rinne, A. & Mutanen, M. (2021) ddRAD Sequencing Sheds Light on Low Interspecific and High Intraspecific mtDNA Divergences in Two Groups of Caddisflies. Insect Systematics and Diversity, 5 (5), 1–10. https://doi.org/10.1093/isd/ixab013
- Schomann, A.M. & Solodovnikov, A. (2017) Phylogenetic placement of the austral rove beetle genus Hyperomma triggers changes in classification of Paederinae (Coleoptera: Staphylinidae). Zoologica Scripta, 46, 336–347. https://doi.org/10.1111/zsc.12209
- Shaikevich, E.V., Ivshina, E.V. & Zakharov, I.A. (2012) Polymorphism of Mitochondrial DNA and Distribution of Cytoplasmic Symbionts in the Populations of Two-Spot Ladybird Beetle Adalia bipunctata. Russian Journal of Genetics, 48 (5), 567–571. https://doi.org/10.1134/S1022795412040102
- Smetana, A. (1958) Fauna ČSR. Svazek 12. Drabčíkovití – Staphylinidae I. Staphylinae (Coleoptera). [Fauna of the ČSR. Vol. 12. Rove beetles -- Staphylinidae I. Staphylinae (Coleoptera)]. Československá akademie věd, Praha, 435 pp. [in Czech]
- Smetana, A. (1971) Revision of the tribe Quediini of America north of Mexico (Coleoptera: Staphylinidae). Memoirs of the Entomological Society of Canada, 79, 1–303. https://doi.org/10.4039/Ent1031833-12
- Smetana, A. (1981) Revision of the tribe Quediini of America North of Mexico (Coleoptera: Staphylinidae). Supplementum 5. The Canadian Entomologist, 113, 631–644. https://doi.org/10.4039/Ent113631-7
- Smetana, A. (2003) Quedius (Quedius) sundukovi (Coleoptera, Staphylinidae, Staphylinini, Quediina), an interesting new species from the Russian Far East. Elytra, 31, 189–193.
- Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Stephens, J.F. (1829) The nomenclature of British insects: being a compendious list of such species as are contained in the systematic catalogue of British insects and forming a guide to their classification. Baldwin and Cradock, London, 68 pp. https://doi.org/10.5962/bhl.title.51800
- Stephens, J.F. (1832) Illustrations of British entomology; or a synopsis of indigenous insects; containing their generic and specific distinctions; with an account of their metamorphoses, times of appearance, localities, food, and economy, as far as practicable. Vol. 5. Mandibulata. Baldwin & Cradock, London, 240 PP.
- Tamura, K. & Kumar, S. (2002) Evolutionary distance estimation under heterogeneous substitution pattern among lineages. Molecular Biology and Evolution, 19, 1727–1736. https://doi.org/10.1093/oxfordjournals.molbev.a003995
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. https://doi.org/10.1093/molbev/mst197
- Tokareva, A.S., Konstantinov, F.V., Brunke, A.J. & Solodovnikov, A. (2021) DNA-barcode and endophallus morphology delimit congruent species in a systematic revision of the oxyporine rove beetles of Russia (Coleoptera: Staphylinidae: Oxyporinae). Contributions to Zoology, 90, 344–407. https://doi.org/10.1163/18759866-bja10020
- Toussaint, E.F.A., Morinière, J., Müller, C.J., Kunte, K., Turlin, B., Hausmann, A. & Balke, M. (2015) Comparative molecular species delimitation in the charismatic Nawab butterflies (Nymphalidae, Charaxinae, Polyura). Molecular Phylogenetics and Evolution, 91, 194–209. https://doi.org/10.1016/j.ympev.2015.05.015
- Yoo, I.S., Frank, J.H., Jung, J.K. & Ahn, K.J. (2022) Integrative taxonomy of coastal Cafius bistriatus (Erichson) species complex (Coleoptera, Staphylinidae). ZooKeys, 1100, 57–70. https://doi.org/10.3897/zookeys.1100.79435
- Zakharov, I. & Shaikevich, E. (2013) Cpmparative study of mtDNA in species of the genus Adalia (Coleoptera: Cocinellidae) and origin of anecient mitochondrial haplotypes in the gene pool of Adalia bupunctata. European Journal of Entomology, 110 (3), 427-433. [http://wwweje.cz/pdfs/110/3/427] https://doi.org/10.14411/eje.2013.057