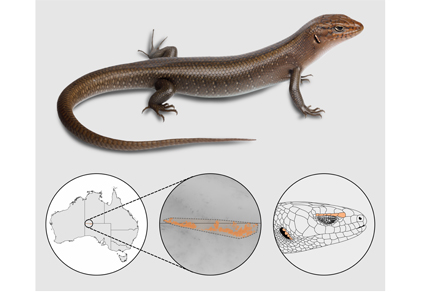
Abstract
A new species of rock skink Liopholis Fitzinger 1843 (Scincidae) is described from the Mann-Musgrave Ranges of north-western South Australia. Liopholis margaretae sensu lato (Storr 1968) is currently known to occur in two disjunct populations: the MacDonnell Ranges bioregion and nearby regions in the Northern Territory, and the Central Ranges bioregion in South Australia. Based on morphological examination of both museum and field specimens, as well as on newly generated molecular data, we show that specimens from these two ranges constitute distinct species. The new species, Liopholis aputja sp. nov. is endemic to a specific geological landform (Mann-Musgrave Ranges) within the Central Ranges bioregion, and is estimated to have diverged from the MacDonnell Ranges population during widespread aridification of the Miocene. Liopholis aputja sp. nov. is distinguished from congeners by a combination of its moderately large size (SVL up to 135 mm), relatively pointed snout, smooth dorsal scales, black and calloused subdigital lamellae and soles of feet, and possessing usually six supraciliary scales and usually four enlarged ear lobules. This new species adds to the list of endemic herpetofauna recognised in the Central Ranges bioregion.
References
- ASH (2022) Position of the Australian Society of Herpetologist on the increasing proliferation of names for taxa without adequate diagnosis or description and published without the benefits of peer review. ASH, Position Statement Version 3. 18 January 2022. Australian Society of Herpetologists Inc., Fairbridge, Western Australia. Available from: https://keyboard-kumquat-lkg2.squarespace.com/s/ASH_Taxonomic_Position_Statement_18-Jan-2022.pdf (accessed 28 April 2024)
- ASH (2023) Australian Society of Herpetologists Official List of Australian Species. Australian Society of Herpetologists Inc., Fairbridge, Western Australia. Available from: https://www.australiansocietyofherpetologists.org/ash-official-list-of-australian-species (accessed 28 April 2024)
- Brennan, I.G., Chapple, D.G., Keogh, J.S. & Donnellan, S. (2024) Evolutionary bursts drive morphological novelty in the world’s largest skinks. Current Biology, 34 (17), 3905–3916. https://doi.org/10.1016/j.cub.2024.07.039
- Byrne, M. (2008) Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quaternary Science Reviews, 27 (27–28), 2576–2585. https://doi.org/10.1016/j.quascirev.2008.08.032
- Byrne, M., Yeates, D.K., Joseph, L., Kearney, M., Bowler, J., Williams, M.A.J., Cooper, S., Donnellan, S.C., Keogh, J.S., Leys, R. & Melville, J. (2008) Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology, 17 (20), 4398–4417. https://doi.org/10.1111/j.1365-294X.2008.03899.x
- Chan, K.O. & Grismer, L.L. (2022) GroupStruct: an R package for allometric size correction. Zootaxa, 5124 (4), 471–482. https://doi.org/10.11646/zootaxa.5124.4.4
- Chang, C., Wu, P., Baker, R.E., Maini, P.K., Alibardi, L. & Chuong, C.-M. (2009) Reptile scale paradigm: evodevo, pattern formation and regeneration. The International Journal of Developmental Biology, 53, 813–826. https://doi.org/10.1387/ijdb.072556cc
- Chapple, D.G. & Keogh, J.S. (2004) Parallel adaptive radiations in arid and temperate Australia: molecular phylogeography and systematics of the Egernia whitii (Lacertilia: Scincidae) species group. Biological Journal of the Linnean Society, 83 (2), 157–173. https://doi.org/10.1111/j.1095-8312.2004.00378.x
- Chapple, D.G., Keogh, J.S. & Hutchinson, M.N. (2005) Substantial genetic substructuring in southeastern and alpine Australia revealed by molecular phylogeography of the Egernia whitii (Lacertilia: Scincidae) species group. Molecular Ecology, 14 (5), 1279–1292. https://doi.org/10.1111/j.1365-294X.2005.02463.x
- Clarke, K.R. (1993) Non‐parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18 (1), 117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
- Cogger, H. (2014) Reptiles and amphibians of Australia. CSIRO publishing, 1096 pp. https://doi.org/10.1071/9780643109773
- Donnellan, S.C., Mahony, M. & Bertozzi, T. (2012) A new species of Pseudophryne (Anura: Myobatrachidae) from the central Australian ranges. Zootaxa, 3476 (1), 69–85. https://doi.org/10.11646/zootaxa.3476.1.4
- de Queiroz, K. (1998) The general lineage concept of species, species criteria, and the process of speciation: A conceptual unification and terminological recommendations. In: Howard, D.J. & Berlocher, S.H. (Eds.), Endless Forms: Species and Speciation. Oxford University Press, Oxford, pp. 57–75.
- de Queiroz K. (2007) Species concepts and species delimitation. Systematic Biology, 56 (6), 879–886. https://doi.org/10.1080/10635150701701083
- Donnellan, S.C., Mahony, M. & Bertozzi, T. (2012) A new species of Pseudophryne (Anura: Myobatrachidae) from the central Australian ranges. Zootaxa, 3476 (1), 69–85. https://doi.org/10.11646/zootaxa.3476.1.4
- Doughty, P., Ellis, R.J. & Oliver, P.M. (2016) Many things come in small packages: revision of the clawless geckos (Crenadactylus: Diplodactylidae) of Australia. Zootaxa, 4168 (2), 239–278. https://doi.org/10.11646/zootaxa.4168.2.2
- Edgoose, C.J. (2012) The Amadeus Basin, Central Australia. Episodes Journal of International Geoscience, 35 (1), 256–263. https://doi.org/10.18814/epiiugs/2012/v35i1/025
- Fenner, A., McDonald, P., Chapple, D.C, Hutchinson, M. & Cogger, H. (2018) Liopholis margaretae. The IUCN Red List of Threatened Species, 2018, e.T109478464A109478481. Available from: https://www.iucnredlist.org/species/109478464/109478481 (accessed 28 April 2024)
- Gardner, M.G., Hugall, A.F., Donnellan, S.C., Hutchinson, M.N. & Foster, R. (2008) Molecular systematics of social skinks: phylogeny and taxonomy of the Egernia group (Reptilia: Scincidae). Zoological Journal of the Linnean Society, 154 (4), 781–794. https://doi.org/10.1111/j.1096-3642.2008.00422.x
- Guttman, L. (1954) Some necessary conditions for common-factor analysis. Psychometrika, 19 (2), 149–161. https://doi.org/10.1007/BF02289162
- Henzell, R.P. (1972) Adaptation to aridity in lizards of the Egernia whitei species-group. Ph.D. Thesis, University of Adelaide, Adelaide. [published online]
- Hoang, D.T., Chernomor, O., Von Haeseler, A., Minh, B.Q. & Vinh, L.S. (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution, 35 (2), 518–522. https://doi.org/10.1093/molbev/msx281
- Horner, P. (1992) Skinks of the Northern Territory. Northern Territory Museum of Arts and Sciences Handbook Series No. 2. Northern Territory Museum of Arts and Sciences, Darwin, 174 pp.
- IUCN (2012) IUCN Red List Categories and Criteria. Version 3.1. 2nd Edition. IUCN Species Survival Commission, IUCN, Gland and Cambridge, iv + 32 pp. Available from: https://portals.iucn.org/library/node/10315 (accessed 28 April 2024)
- IUCN (2022) The Guidelines for Using the IUCN Red List Categories and Criteria. Version 15.1. July 2022. Available from: https://www.iucnredlist.org/resources/redlistguidelines (accessed 28 April 2024)
- Jackson, D.A. (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology, 74 (8), 2204–2214. https://doi.org/10.2307/1939574
- Johnston, G.R. (1992) Relictual population of Tiliqua scincoides (Sauria: Scincidae) in north-western South Australia. Transactions of the Royal Society of South Australia, 116, 149–150. https://doi.org/10.2307/1939574
- Jolly, C., Schembri, B. & Macdonald, S. (2023) Field Guide to the Reptiles of the Northern Territory. CSIRO Publishing, Melbourne, 424 pp. https://doi.org/10.1071/9781486312696
- Kaiser, H., Crother, B.I., Kelly, C.M., Luiselli, L., O’Shea, M., Ota, H., Passos, P., Schleip, W.D. & Wüster, W. (2013) Best practices: in the 21st century, taxonomic decisions in herpetology are acceptable only when supported by a body of evidence and published via peer-review. Herpetological Review, 44 (1), 8–23.
- Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K., Von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14 (6), 587–589. https://doi.org/10.1038/nmeth.4285
- Kassambara, A. & Mundt, F. (2020) Factoextra: Extract and visualize the results of multivariate data analyses. R Package Version 1.0.7. Available from: https://CRAN.R-project.org/package=factoextra (accesed 28 April 2024)
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30 (4), 772–780. https://doi.org/10.1093/molbev/mst010
- Kleiber, C. & Zeileis, A. (2008) AER: Applied Econometrics with R. R package Version 1.2–10. Available from: https://CRAN.R-project.org/package=AER (accessed 28 April 2024) https://doi.org/10.32614/CRAN.package.AER
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35 (6), 1547. https://doi.org/10.1093/molbev/msy096
- Lê, S., Josse, J. & Husson, F. (2008) FactoMineR: an R package for multivariate analysis. Journal of Statistical Software, 25, 1–18. https://doi.org/10.18637/jss.v025.i01
- Lleonart, J., Salat, J. & Torres, G.J. (2000) Removing allometric effects of body size in morphological analysis. Journal of Theoretical Biology, 205 (1), 85–93. https://doi.org/10.1006/jtbi.2000.2043
- Martin, H.A. (2006) Cenozoic climatic change and the development of the arid vegetation in Australia. Journal of Arid Environments, 66 (3), 533–563. https://doi.org/10.1016/j.jaridenv.2006.01.009
- McCoy, M.W., Bolker, B.M., Osenberg, C.W., Miner, B.G. & Vonesh, J.R. (2006) Size correction: Comparing morphological traits among populations and environments. Oecologia, 148, 547–554. https://doi.org/10.1007/s00442-006-0403-6
- McDonald, P.J., Jobson, P., Köhler, F., Nano, C.E. & Oliver, P.M. (2021) The living heart: Climate gradients predict desert mountain endemism. Ecology and Evolution, 11 (9), 4366–4378. https://doi.org/10.1002/ece3.7333
- Melville, J., Haines, M.L., Hale, J., Chapple, S. & Ritchie, E.G. (2016) Concordance in phylogeography and ecological niche modelling identify dispersal corridors for reptiles in arid Australia. Journal of Biogeography, 43 (9), 1844–1855. https://doi.org/10.1111/jbi.12739
- Moore, D., Stow, A. & Kearney, M.R. (2018) Under the weather?—The direct effects of climate warming on a threatened desert lizard are mediated by their activity phase and burrow system. Journal of Animal Ecology, 87 (3), 660–671. https://doi.org/10.1111/1365-2656.12812
- Nguyen, L.T., Schmidt, H.A., Von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32 (1), 268–274. https://doi.org/10.1093/molbev/msu300
- Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E. & Wagner, H. (2020) Vegan: Community Ecology Package. Available from: https://CRAN.R-project.org/package=vegan (accessed 28 April 2024)
- Oliver, P.M. & Bauer, A.M. (2011) Systematics and evolution of the Australian knob-tail geckos (Nephrurus, Carphodactylidae, Gekkota): plesiomorphic grades and biome shifts through the Miocene. Molecular Phylogenetics and Evolution, 59 (3), 664–674. https://doi.org/10.1016/j.ympev.2011.03.018
- Oliver, P.M. & McDonald, P.J. (2016) Young relicts and old relicts: a novel palaeoendemic vertebrate from the Australian Central Uplands. Royal Society Open Science, 3 (10), 160018. https://doi.org/10.1098/rsos.160018
- Oliver, P.M., Adams, M. & Doughty, P. (2010) Molecular evidence for ten species and Oligo-Miocene vicariance within a nominal Australian gecko species (Crenadactylus ocellatus, Diplodactylidae). BMC Evolutionary Biology, 10, 1–11. https://doi.org/10.1186/1471-2148-10-386
- Oliver, P.M., Donnellan, S.C. & Gunn, B.F. (2022) Plio-Pleistocene vicariance across arid Australia in the ‘Spiny Knob-tailed Geckos’ (Nephrurus asper group), with the description of a new species from western Queensland. Australian Journal of Zoology, 69 (6), 216–228. https://doi.org/10.1071/ZO22008
- Pavey, C. (2004) Recovery plan for Slater’s skink, Egernia slateri, 2005–2010. Northern Territory Department of Infrastructure, Planning and Environment, Darwin, Northern Territory. Available from: https://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=83163 (accessed 28 April 2024)
- Pepper, M. & Keogh, J.S. (2021) Life in the “dead heart” of Australia: The geohistory of the Australian deserts and its impact on genetic diversity of arid zone lizards. Journal of Biogeography, 48 (4), 716–746. https://doi.org/10.1111/jbi.14063
- Pepper, M., Doughty, P., Fujita, M.K., Moritz, C. & Keogh, J.S. (2013) Speciation on the rocks: integrated systematics of the Heteronotia spelea species complex (Gekkota; Reptilia) from western and central Australia. PLoS ONE, 8 (11), e78110. https://doi.org/10.1371/journal.pone.0078110
- Pepper, M., Fujita, M.K., Moritz, C. & Keogh, J.S. (2011) Palaeoclimate change drove diversification among isolated mountain refugia in the Australian arid zone. Molecular Ecology, 20 (7), 1529–1545. https://doi.org/10.1111/j.1365-294X.2011.05036.x
- R Core Team (2024) R: a language and environment for statistical computing. Version 4.3.1. R Foundation for Statistical Computing, Vienna. Available from: https://www.R-project.org/ (accessed 24 September 2024)
- Rabosky, D.L., Donnellan, S.C., Talaba, A.L. & Lovette, I.J. (2007) Exceptional among-lineage variation in diversification rates during the radiation of Australia’s most diverse vertebrate clade. Proceedings of the Royal Society B: Biological Sciences, 274 (1628), 2915–2923. https://doi.org/10.1098/rspb.2007.0924
- Reist, J.D. (1985) An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Canadian Journal of Zoology, 63, 1429–1439. https://doi.org/10.1139/z85-213
- Robinson, A.C., Copley, P.B., Canty, P.D., Baker, L.M. & Nesbitt, B.J. (2003) A biological survey of the Anangu Pitjantjatjara lands, South Australia, 1998–2001. Department for Environment and Heritage, South Australia. Available from: https://data.environment.sa.gov.au/Content/Publications/Anangu-Pitjantjatjara-Lands-BioSurvey.pdf (accessed 28 April 2024)
- Shoo, L.P., Rose, R., Doughty, P., Austin, J.J. & Melville, J. (2008) Diversification patterns of pebble-mimic dragons are consistent with historical disruption of important habitat corridors in arid Australia. Molecular Phylogenetics and Evolution, 48 (2), 528–542. https://doi.org/10.1016/j.ympev.2008.03.022
- Sistrom, M., Donnellan, S.C., Hutchinson, M.N. (2013) Delimiting species in recent radiations with low levels of morphological divergence: A case study in Australian Gehyra geckos. Molecular Phylogenetics and Evolution, 68 (1), 135–143. https://doi.org/10.1016/j.ympev.2013.03.007
- Storr, G.M. (1968) Revision of the Egernia whitei species-group (Lacertilia, Scincidae). Journal of the Royal Society of Western Australia, 51 (2), 51–62.
- Taxonomy Australia (2024) “Our Position on Taxonomic Vandalism”. Available from: https://www.taxonomyaustralia.org.au/codes-of-conduct (accessed 17 July 2024)
- Thackway, R. & Cresswell, I.D. (1995) An Interim Biogeographic Regionalisation for Australia: a framework for establishing the national system of reserves. Version 4.0. Australian Nature Conservation Agency, Canberra, 88 pp. Available from: https://www.dcceew.gov.au/sites/default/files/documents/ibra-framework-setting-priorities-nrs-cooperative-program.pdf (accessed 28 April 2024)
- Thorpe, R.S. (1975) Quantitative handling of characters useful in snake systematics with particular reference to intraspecific variation in the ringed snake Natrix natrix (L.). Biological Journal of the Linnean Society, 7 (1), 27–43. https://doi.org/10.1111/j.1095-8312.1975.tb00732.x
- Thorpe, R.S. (1983) A review of the numerical methods for recognising and analysing racial differentiation. In: Felsenstein, J. (Eds.), Numerical Taxonomy. NATO ASI Series. Vol. 1. Springer, Berlin/Heidelberg, pp. 404–423. https://doi.org/10.1007/978-3-642-69024-2_43
- Title, P.O. & Bemmels, J.B. (2018) ENVIREM: an expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography, 41 (2), 291–307. https://doi.org/10.1111/ecog.02880
- Turan, C. (1999) A note on the examination of morphometric differentiation among fish populations: the truss system. Turkish Journal of Zoology, 23 (3), 259–264.
- Wilson, S. & Swan, G. (2021) A Complete Guide to Reptiles of Australia. 6th Edition. Reed New Holland, Wahroonga, New South Wales, 572 pp.
- Wüster, W., Thomson, S.A., O’Shea, M. & Kaiser, H. (2021) Confronting taxonomic vandalism in biology: conscientious community self-organization can preserve nomenclatural stability. Biological Journal of the Linnean Society, 133 (3), 645–670. https://doi.org/10.1093/biolinnean/blab009
- Zimmerman, D.W. (2004) A note on preliminary tests of equality of variances. British Journal of Mathematical and Statistical Psychology, 57 (1), 173–181. https://doi.org/10.1348/000711004849222