Abstract
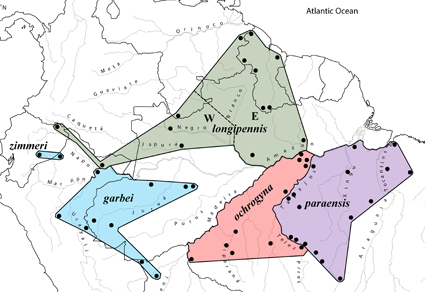
We examined DNA sequences of 328 individuals of three lineages (species or species complexes) of Amazonian antwrens to evaluate their degree of geographical and historical concordance. All lineages (Myrmotherula longipennis, M. menetriesii, and the Isleria guttata-hauxwelli species complex) consist of small insectivorous birds that occupy understory or midstory of terra firme forest and are widely distributed across the Amazon Basin. Individuals of each of the three lineages grouped into genetic clades mainly separated by the Amazon and some major tributaries, although members of different clades of both M. menetriesii and I. hauxwelli were documented in the Madeira-Tapajós interfluvium. Branching patterns differed among taxa, but all taxa were highly differentiated across the lower or upper Amazon. Despite the morphological, ecological, and phylogenetic similarities among lineages, and despite the fact that nearly all taxa are bounded by rivers, the effect of the other major rivers as biogeographic barriers was highly variable. The Marañón, Ucayali, Madeira, Tapajós, Xingu, Napo, Negro, and Branco all separated main clades in one or two lineages but not in the others. Levels of genetic differentiation along the Teles Pires were substantially higher than those across the Tapajós in M. menetriesii and I. hauxwelli, consistent with a proposed historical change of river course for the Tapajós. Genetic units in this study were comparable for the most part to taxonomic units delineated by analyses of vocal and morphological variation, and identical, with one exception, to units defined solely by vocal variation in a companion paper (Isler et al. 2025). These results, in conjunction with those of Isler et al. (2025), provide additional instances of the variability of responses to the historical dynamism of Amazonia, in this case in closely related and ecologically similar species; highlight the consistency of genetic differentiation with vocal differentiation in additional species of Neotropical suboscine birds; support the importance of Amazonian rivers in creating conditions that result in the differentiation of independent evolutionary lineages; and demonstrate that species richness in two of the lineages studied (M. longipennis and M. menetriesii) was previously underestimated.
References
- Aleixo, A. (2004) Historical diversification of a terra-firme forest bird superspecies: a phylogeographic perspective on the role of different hypotheses of Amazonian diversification. Evolution, 58, 1303–1317. https://doi.org/10.1111/j.0014-3820.2004.tb01709.x
- Ayres, J.M. & Clutton-Brock, T.H. (1992) River boundaries and species range size in Amazonian primates. American Naturalist, 140, 531–537. https://doi.org/10.1086/285427
- Ballard, J.W.O. & Whitlock, M.C. (2004) The incomplete natural history of mitochondria. Molecular Ecology, 13, 729–744. https://doi.org/10.1046/j.1365-294X.2003.02063.x
- Baptista, L. & Kroodsma, D. (2001) Avian Bioacoustics. In: del Hoyo, J., Elliot, A. & Sargatal, J. (Eds.), Handbook of the Birds of the World. Vol. 6. Lynx Edicions, Barcelona, pp. 11–52.
- Barrera-Guzman, A.O., Aleixo, A., Shawkey, M.D. & Weir, J.T. (2018) Hybrid speciation leads to novel male secondary sexual ornamentation of an Amazonian bird. Proceedings of the National Academy of Sciences, E218–E225. https://doi.org/10.1073/pnas.1717319115
- Bates, J.M., Haffer, J. & Grismer, E. (2004) Avian mitochondrial DNA sequence divergence across a headwater stream of the Rio Tapajós, a major Amazonian river. Journal of Ornithology, 145, 199–205. https://doi.org/10.1007/s10336-004-0039-4
- Berlepsch, H. von & Hartert, E. (1902) On the birds of the Orinoco region. Novitates Zoologicae, 9, 1–134.
- Boubli, J.P., Ribas, C., Alfaro, J.W.L., Alfaro, M.E., Silva, M.N.F. & Pinho, G.M. (2015) Spatial and temporal patterns of diversification on the Amazon: A test of the riverine hypothesis for all diurnal primates of Rio Negro and Rio Branco in Brazil. Molecular Phylogenetics and Evolution, 82, 400–412. https://doi.org/10.1016/j.ympev.2014.09.005
- Bouckaert, R., Heled, J., Kühnert, D., Vaughan, T., Wu, C.-H., Xie, D., Suchard, M.A., Rambaut, A. & Drummond, A.J. (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10, e1003537. https://doi.org/10.1371/journal.pcbi.1003537
- Bravo, G.A. (2012) Phenotypic and niche evolution in the antbirds (Aves, Thamnophilidae). Ph.D dissertation. Louisiana State University, Baton Rouge, Louisiana, 169 pp.
- Burney, C.W. & Brumfield, R.T. (2009) Ecology predicts levels of genetic differentiation in Neotropical birds. American Naturalist, 174, 358–368. https://doi.org/10.1086/603613
- Chapman, F.M. (1925) Descriptions of one new genus and of species of birds from Peru and Ecuador. American Museum Novitates, 205, 1–11.
- Chesser, R.T. (1999) Molecular systematics of the rhinocryptid genus Pteroptochos. Condor, 101, 439–446. https://doi.org/10.2307/1370012
- Cheviron, Z., Hackett, S.J. & Capparella, A.P. (2005) Complex evolutionary history of a Neotropical lowland forest bird (Lepidothrix coronata) and its implications for historical hypotheses of the origin of Neotropical avian diversity. Molecular Phylogenetics and Evolution, 36, 338–357. https://doi.org/10.1016/j.ympev.2005.01.015
- Claramunt, S., Hong, M. & Bravo, A. (2022) The effect of flight efficiency on gap-crossing ability in Amazonian forest birds. Biotropica, 54, 860–868. https://doi.org/10.1111/btp.13109
- Clements, J.F., Rasmussen, P.C., Schulenberg, T.S., Iliff, M.J., Fredericks, T.A., Gerbracht, J.A., Lepage, D., Spencer, A., Billerman, S.M., Sullivan, B.L. & Wood, C.L. (2023) The eBird/Clements Checklist of Birds of the World. Version 2023b. Available from: https://www.birds.cornell.edu/clementschecklist/download/ (accessed 14 July 2025)
- Cohn-Haft, M., Pacheco, A.M.F., Bechtoldt, C.L., Torres, M.F.N.M., Fernandes, A.M., Sardelli, C.H. & Macêdo, I.T. (2007) n.k. In: Py-Daniel, L.R., Deus, C.P., Henriques, A.L., Pimpão, D.M. & Ribeiro, M.O. (Eds.), Inventário Ornitológico, Biodiversidade do Médio Madeira: Bases Científicas para Propostas de Conservação. INPA, Manaus, pp. 145–178.
- Cracraft, J. (1985) Historical Biogeography and Patterns of Differentiation within South American Birds: Areas of Endemism. In: Buckley, P.A., Foster, M.S., Morton, E.S., Ridgely, R.S. & Buckley, F.G. (Eds.), Neotropical Ornithology. Ornithological Monographs, No. 36, pp. 49–84. https://doi.org/10.2307/40168278
- Cracraft, J. & Prum, R.O. (1988) Patterns and processes of diversification: Speciation and historical congruence in some neotropical birds. Evolution, 42, 603–620. https://doi.org/10.2307/2409043
- Cracraft, J., Camila Ribas, C., d’Horta, F.M., Bates, J., Almeida, R.P., Aleixo, A., Boubli, J.P., Campbell, K.E., Cruz, F.W., Ferreira, M., Fritz, S.C., Grohmann, C.H., Latrubesse, E.M., Lohmann, L.G., Musher, L.J., Nogueira, A., Sawakuchi, A.O. & Baker, P. (2020) The Origin and Evolution of Amazonian Species Diversity. In: Rull, V. & Carnaval, A.C. (Eds.), Neotropical Diversification: Patterns and Processes. Springer International Publishing, Cham, pp. 225–244. https://doi.org/10.1007/978-3-030-31167-4_10
- Cronemberger, Á.A., Aleixo, A., Mikkelson, E.K. & Weir, J.T. (2020) Postzygotic isolation drives genomic speciation between highly cryptic Hypocnemis antbirds from Amazonia. Evolution, 74, 2512–2525. https://doi.org/10.1111/evo.14103
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9 (8), 772. https://doi.org/10.1038/nmeth.2109
- Del-Rio, G., Rego, M.A., Whitney, B.M., Schunck, F., Silveira, L.F., Faircloth, B.C. & Brumfield, R.T. (2022) Displaced clines in an avian hybrid zone (Thamnophilidae: Rhegmatorhina) within an Amazonian interfluve. Evolution, 76, 455–475. https://doi.org/10.1111/evo.14377
- Dias, C., Lima, K.A., Araripe, J., Aleixo, A., Vallinoto, M., Sampaio, I., Schneider, H. & Rêgo, P.S. (2018) Mitochondrial introgression obscures phylogenetic relationships among manakins of the genus Lepidothrix (Aves: Pipridae). Molecular Phylogenetics and Evolution, 126, 314–320. https://doi.org/10.1016/j.ympev.2018.04.017
- Dickinson, E.C. & Christidis, L. (Eds.) (2014) The Howard and Moore Complete Checklist of the Birds of the World. Vol. 2. Passerines. 4th Edition. Aves Press, Eastbourne, 752 pp.
- d’Orbigny, A.D. (1838) Voyage dans l’Amérique Méridionale. Livr. 30. Oiseaux. Bertrand & Levrault, Strasbourg, 184 pp. [citation corrected in Dickinson & Lebossé (2018) pp. 86]
- Fernandes, A.M. (2013) Fine-scale endemism of Amazonian birds in a threatened landscape. Biodiversity Conservation, 22, 2683–2694. https://doi.org/10.1007/s10531-013-0546-9
- Fernandes, A.M., Wink, M. & Aleixo, A. (2012) Molecular phylogeography of the Chestnut-tailed Antbird (Myrmeciza hemimelaena) clarifies the role of rivers in Amazonian biogeography. Journal of Biogeography, 39, 1524–1535. https://doi.org/10.1111/j.1365-2699.2012.02712.x
- Fernandes, A.M., Gonzalez, J., Wink, M. & Aleixo, A. (2013) Multilocus phylogeography of the Wedge-billed Woodcreeper Glyphorynchus spirurus (Aves, Furnariidae) in lowland Amazonia: Widespread cryptic diversity and paraphyly reveal a complex diversification pattern. Molecular Phylogenetics and Evolution, 66, 270–282. https://doi.org/10.1016/j.ympev.2012.09.033
- Fernandes, A.M., Cohn-Haft, M., Hrbek, T. & Farias, I.P. (2014) Rivers acting as barriers for bird dispersal in the Amazon. Revista Brasileira de Ornitologia, 22, 363–373. https://doi.org/10.1007/BF03544273
- Ferreira, M., Fernandes, A.M., Aleixo, A., Antonelli, A., Olsson, U., Bates, J.M., Cracraft, J. & Ribas, C.C. (2018) Evidence for mtDNA capture in the jacamar Galbula leucogastra/chalcothorax species-complex and insights on the evolution of white-sand ecosystems in the Amazon basin. Molecular Phylogenetics and Evolution, 129, 149–157. https://doi.org/10.1016/j.ympev.2018.07.007
- Fjeldså, J. & Krabbe, N. (1990) Birds of the High Andes. Zoological Museum, University of Copenhagen, Copenhagen, 876 pp.
- Funk, D.J. & Omland, K. (2003) Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology Evolution and Systematics, 34, 397–423. https://doi.org/10.1146/annurev.ecolsys.34.011802.132421
- Guindon, S. & Gascuel, O. (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology, 52, 696–704. https://doi.org/10.1080/10635150390235520
- Haffer, J. (1969) Speciation in Amazonian forest birds. Science, 165, 131–137. https://doi.org/10.1126/science.165.3889.131
- Haffer, J. (1974) Avian Speciation in Tropical South America. With a systematic survey of the toucans (Ramphastidae) and jacamars (Galbulidae). Publications of the Nuttall Ornithological Club, No. 14, 1–390.
- Haffer, J. (1978) Distribution of Amazonian forest birds. Bonner Zoologische Beiträge, 29, 38–78.
- Haffer, J. (1985) Avian zoogeography of the Neotropical lowlands. In: Buckley, P.A., Foster, M.S., Morton, E.S., Ridgely, R.S. & Buckley, F.G. (Eds.), Neotropical Ornithology. Ornithological Monographs, No. 36, pp. 113–146.
- Harris, R.J. & Reed, J.M. (2002) Behavioral barriers to nonmigratory movements of birds. Annales Zoologici Fennici, 39, 275–290.
- Harvey, M.G., Seeholzer, G.F., Cáceres, Á.D., Winger, B.M. & Tello, J.G. (2014) The avian biogeography of an Amazonian headwater: The Upper Ucayali River, Peru. Wilson Journal of Ornithology, 126, 179–191. https://doi.org/10.1676/13-135.1
- Harvey, M.G., Aleixo, A., Ribas, C.C. & Brumfield, R.T. (2017) Habitat association predicts genetic diversity and population divergence in Amazonian birds. American Naturalist, 190, 631–648. https://doi.org/10.1086/693856
- Harvey, M.G., Bravo, G.A., Claramunt, S., Cuervo, A.M., Derryberry, G.E., Battilana, J., Seeholzer, G.F., McKay, J.S., O’Meara, B.C., Faircloth, B.C., Edwards, S.V., Pérez-Emán, J., Moyle, R.G., Sheldon, F.H., Aleixo, A., Smith, B.T., Chesser, R.T., Silveira, L.F., Cracraft, J., Brumfield, R.T. & Derryberry, E.P. (2020) The evolution of a tropical biodiversity hotspot. Science, 370, 343–348. https://doi.org/10.1126/science.aaz6970
- Hawkins, T.L., O’Connor-Morin, T., Roy, A. & Santillan, D. (1994) DNA purification and isolation using a solid‐phase. Nucleic Acids Research, 22, 4543–4544. https://doi.org/10.1093/nar/22.21.4543
- Hayes, F.E. & Sewlal, J.N. (2004) The Amazon River as a dispersal barrier to passerine birds: effects of river width, habitat and taxonomy. Journal of Biogeography, 31, 1809–1818. https://doi.org/10.1111/j.1365-2699.2004.01139.x
- Helm-Bychowski, K. & Cracraft, J. (1993) Recovering phylogenetic signal from DNA sequences: Relationships within the corvine assemblage (class Aves) as inferred from complete sequences of the mitochondrial DNA cytochrome-b gene. Molecular Biology and Evolution, 10 (6), 1196–1214.
- Hellmayr, C.E. (1903) Über neue und wenig bekannte sudamerikanische Vögel. Verhandlungen der k. k. zoologisch-botanischen Gesellschaft in Wien, 53, 199–223.
- Hellmayr, C.E. (1910) The birds of the Rio Madeira. Novitates Zoologicae, 17, 257–428. https://doi.org/10.5962/bhl.title.875
- Hellmayr, C.E. (1929) On heterogynism in formicarian birds. Journal für Ornithologie Festschrift für Ernst Hartert, 41–70. https://doi.org/10.1007/BF01917232
- Ihering, H. von (1905) O rio Juruá. Revista do Museu Paulista, 6, 385–460.
- Isler, M.L., Chesser, R.T., Stryjewski, K.F. & Whitney, B.M. (2025) Systematics of three pan-Amazonian antwren lineages (Aves: Passeriformes: Thamnophilidae: Myrmotherula and Isleria). Zootaxa, 5722 (1), 45–78. https://doi.org/10.11646/zootaxa.5722.1.2
- Isler, M.L., Cuervo, A.M., Bravo, G.A. & Brumfield, R.T. (2012) An integrative approach to species-level systematics reveals the depth of diversification in an Andean thamnophilid, the Long-tailed Antbird. Condor, 114, 571–583. https://doi.org/10.1525/cond.2012.120012
- Isler, M.L., Isler, P.R. & Brumfield, R.T. (2005) Clinal variation in vocalizations of an antbird (Thamnophilidae) and implications for defining species limits. Auk, 122, 433–444. https://doi.org/10.1093/auk/122.2.433
- Isler, M.L., Isler, P.R. & Whitney, B.M. (2007) Species limits in antbirds (Thamnophilidae): the warbling antbird (Hypocnemis cantator) complex. Auk, 124, 11–28. https://doi.org/10.1093/auk/124.1.11
- Isler, M.L. & Maldonado-Coelho, M. (2017) Calls distinguish species of antbirds (Aves: Passeriformes: Thamnophilidae) in the genus Pyriglena. Zootaxa, 4291 (2), 275–294. https://doi.org/10.11646/zootaxa.4291.2.3
- Johnson, K.P. & Sorenson, M.D. (1998) Comparing molecular evolution in two mitochondrial protein coding genes (cytochrome b and ND2) in the dabbling ducks (Tribe: Anatini). Molecular Phylogenetics and Evolution, 10, 82–94. https://doi.org/10.1006/mpev.1997.0481
- Kocher, T.D., Thomas, W.K., Meyer, A., Edwards, S.V., Paabo, S., Villablanca, F.X. & Wilson, A.C. (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proceedings of the National Academy of Sciences of the USA, 86, 6196–6200. https://doi.org/10.1073/pnas.86.16.6196
- Kroodsma, D.E. & Konishi, M. (1991) A suboscine bird (Eastern Phoebe, Sayornis phoebe) develops normal song without auditory feedback. Animal Behavior, 42, 477–487. https://doi.org/10.1016/S0003-3472(05)80047-8
- Marks, B.D., Hackett, S.J. & Capparella, A.P. (2002) Historical relationships among Neotropical lowland forest areas of endemism as determined by mitochondrial DNA sequence variation within the Wedge-billed Woodcreeper (Aves: Dendrocolaptidae: Glyphorynchus spirurus). Molecular Phylogenetics and Evolution, 24, 153–167. https://doi.org/10.1016/S1055-7903(02)00233-6
- Mertes, L.A.K., Dunne, T. & Martinelli, L.A. (1996) Channel-floodplain geomorphology along the Solimões-Amazon River, Brazil. GSA Bulletin, 108, 1089–1107. https://doi.org/10.1130/0016-7606(1996)108%3C1089:CFGATS%3E2.3.CO;2
- Miranda, L.S., Prestes, B.O. & Aleixo, A. (2021) Molecular systematics and phylogeography of a widespread Neotropical avian lineage: evidence for cryptic speciation with protracted gene flow throughout the Late Quaternary. Biological Journal of the Linnean Society, 132, 431–450. https://doi.org/10.1093/biolinnean/blaa193
- Munn, C.A. & Terborgh, J.W. (1979) Multi-species territoriality in Neotropical foraging flocks. Condor, 81, 338–347. https://doi.org/10.2307/1366956
- Musher, L.J., Giakoumis, M., Albert, J., Del-Rio, G., Rego, M., Thom, G., Aleixo, A., Ribas, C.C., Brumfield, R.T., Smith, B.T. & Cracraft, J. (2022) River network rearrangements promote speciation in lowland Amazonian birds. Science Advances, 8, eabn1099. https://doi.org/10.1126/sciadv.abn1099
- Naka, L.N. (2011) Avian distribution patterns in the Guiana Shield: implications for the delimitation of Amazonian areas of endemism. Journal of Biogeography, 38, 681–696. https://doi.org/10.1111/j.1365-2699.2010.02443.x
- Naka, L.N. & Brumfield, R.T. (2018) The dual role of Amazonian rivers in the generation and maintenance of avian diversity. Science Advances, 4, eaar8575. https://doi.org/10.1126/sciadv.aar8575
- Naka, L.N., Bechtoldt, C.L., Henriques, L.M.P. & Brumfield, R.T. (2012) The role of physical barriers in the location of avian suture zones in the Guiana Shield, northern Amazonia. The American Naturalist, 179 (4), E115–E132. https://doi.org/10.1086/664627
- Naka, L.N., Costa, B.M.S., Lima, G.R. & Claramunt, S. (2022) Riverine barriers as obstacles to dispersal in Amazonian birds. Frontiers in Ecology and Evolution, 10, 846975. https://doi.org/10.3389/fevo.2022.846975
- Oliveira, U., Vasconcelos, M.F. & Santos, A.J. (2017) Biogeography of Amazon birds: rivers limit species composition, but not areas of endemism. Scientific Reports, 7, 2992. https://doi.org/10.1038/s41598-017-03098-w
- Parker III, T.A., Schulenberg, T.S., Graves, G.R. & Braun, M.J. (1985) The avifauna of the Huancabamba region, northern Peru. In: Buckley, P.A., Foster, M.S., Morton, E.S., Ridgely, R.S. & Buckley, F.G. (Eds.), Neotropical Ornithology. Ornithological Monographs, No. 36, pp. 169–197. https://doi.org/10.2307/40168282
- Paynter Jr., R.A. & Traylor Jr., M.A. (1991) Ornithological Gazetteer of Brazil. Museum of Comparative Zoology, Cambridge, Massachusetts, 789 pp. https://doi.org/10.5962/bhl.title.14635
- Pelzeln, A. von (1868) Zur Ornithologie Brasiliens, Resultate von Johann Natterers Reisen in den Jahren 1817 bis 1835. Abth. 2. A. Pichler’s Witwe & Sohn, Wien, 462 pp.
- Peters, J.L. (1951) Check-list of the Birds of the World. Vol. 7. Museum of Comparative Zoology, Cambridge, Massachusetts, x + 318 pp.
- Powell, G.V. (1989) On the possible contribution of mixed species flocks to species richness in neotropical avifaunas. Behavioral Ecology and Sociobiology, 24, 387–393. https://doi.org/10.1007/BF00293266
- Quaresma, T.F., Cronemberger, Á.A., Batista, R. & Aleixo, A. (2022) Diversification and species limits in scale-backed antbirds (Willisornis: Thamnophilidae), an Amazonian endemic lineage. Zoological Journal of the Linnaean Society, 196, 1408–1430. https://doi.org/10.1093/zoolinnean/zlac011
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
- Ribas, C.C., Aleixo, A., Nogueira, A.C.R., Miyaki, C.Y. & Cracraft, J. (2012) A palaeobiogeographic model for biotic diversification within Amazonia over the past three million years. Proceedings of the Royal Society of London, Series B Biological Sciences, 279, 681–689. https://doi.org/10.1098/rspb.2011.1120
- Rull, V. (2011) Neotropical biodiversity: timing and potential drivers. Trends in Ecology and Evolution, 26, 508–513. https://doi.org/10.1016/j.tree.2011.05.011
- Rull, V. & Carnaval, A.C. (Eds.) (2020) Neotropical Diversification: Patterns and Processes. Springer International Publishing, Cham, 820 pp. https://doi.org/10.1007/978-3-030-31167-4
- Ruokolainen, K., Moulatlet, G.M., Zuquim, G., Hoorn, C. & Tuomisto, H. (2019) Geologically recent rearrangements in central Amazonian river network and their importance for the riverine barrier hypothesis. Frontiers of Biogeography, 11.3, e45046. https://doi.org/10.21425/F5FBG45046
- Santorelli, S., Magnusson, W.E. & Deus, C.P. (2018) Most species are not limited by an Amazonian river postulated to be a border between endemism areas. Scientific Reports, 8, 2294. https://doi.org/10.1038/s41598-018-20596-7
- Sclater, P.L. (1857) Descriptions of twelve new or little-known species of the South American family Formicariidae. Proceedings of the Zoological Society of London, 1857, 129–133. https://doi.org/10.1111/j.1096-3642.1857.tb01217.x
- Sclater, P.L. & Salvin, O. (1868) Catalogue of birds collected by Mr. E. Bartlett on the River Huallaga, eastern Peru, with notes and descriptions of new species. Proceedings of the Zoological Society of London, 1867, 748–759.
- Sick, H. (1967) Rios e enchentes na Amazonia como obstaculo para a avifauna. In: Lent, H. (Ed.), Atas do Simposio Sobre a Biota Amazonica. Vol. 5. Zoologia. Conselho Nacional de Pesquisas, Rio de Janeiro, pp. 495–520.
- Silva, S.M., Peterson, A.T., Carneiro, L., Burlamaqui, T.C.T., Ribas, C.C., Sousa-Neves, T., Miranda, L.S., Fernandes, A.M., d’Horta, F.M., Araújo-Silva, L.E., Batista, R., Bandeira, C.H.M.M., Dantas, S.M., Ferreira, M., Martins, D.M., Oliveira, J., Rocha, T.C., Sardelli, C.H., Thom, G., Rêgo, P.S., Santos, M.P., Sequiera, F., Vallinoto, M. & Aleixo, A. (2019) A dynamic continental moisture gradient drove Amazonian bird diversification. Science Advances, 5 (7), eaat5752. https://doi.org/10.1126/sciadv.aat5752
- Sioli, H. (1984) The Amazon and its Main Affluents: Hydrography, Morphology of the River Courses, and River Types. In: Sioli, H. (Ed), The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and its Basin. Monographiae Biologicae, No. 56. Dr W. Junk, Dordrecht, The Netherlands, pp. 127–165. https://doi.org/10.1007/978-94-009-6542-3_5
- Smith, B.T., McCormack, J.E., Cuervo, A.M., Hickerson, M.J., Aleixo, A., Cadena, C.D., Pérez-Emán, J., Burney, C.W., Xie, X., Harvey, M.G., Faircloth, B.C., Glenn, T.C., Derryberry, E.P., Prejean, J., Fields, S. & Brumfield, R.T. (2014) The drivers of tropical speciation. Nature, 515, 406–409. https://doi.org/10.1038/nature13687
- Snethlage, E. (1906) Über brasilianische Vögel. Ornithologische Monatsberichte, 14, 9–10.
- Snethlage, E. (1913) Über die verbreitung der vogelarten in unteramazonien. Journal für Ornithologie, 61, 469–539. https://doi.org/10.1007/BF02250374
- Sorenson, M.D., Ast, J.C., Dimcheff, D.E., Yuri, T. & Mindell, D.P. (1999) Primers for a PCR-based approach to mitochondrial genome sequencing in birds and other vertebrates. Molecular Phylogenetics and Evolution, 12, 105–114. https://doi.org/10.1006/mpev.1998.0602
- Swofford, D.L. (2002) PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. [program]
- Thom, G. & Aleixo, A. (2015) Cryptic speciation in the White-shouldered Antshrike (Thamnophilus aethiops, Aves—Thamnophilidae): The tale of a transcontinental radiation across rivers in lowland Amazonia and the northeastern Atlantic Forest. Molecular Phylogenetics and Evolution, 82, 95–110. https://doi.org/10.1016/j.ympev.2014.09.023
- Thom, G., Moreira, L.R., Batista, R., Gehara, M., Aleixo, A. & Smith, B.T. (2024) Genomic architecture predicts tree topology, population structuring, and demographic history in Amazonian birds. Genome Biology and Evolution, 16, evae002. https://doi.org/10.1093/gbe/evae002
- Todd, W.E.C. (1920) Descriptions of apparently new South American birds. Proceedings of the Biological Society of Washington, 33, 71–76.
- Todd, W.E.C. (1927) New gnateaters and antbirds from tropical America, with a revision of the genus Myrmeciza and its allies. Proceedings of the Biological Society of Washington, 40, 149–178.
- Touchton, J.M., Seddon, N. & Tobias, J.A. (2014) Captive rearing experiments confirm song development without learning in a tracheophone suboscine bird. PLoS ONE, 9 (4), e95746. https://doi.org/10.1371/journal.pone.0095746
- Vieillot, L.P. (1825) La galerie des oiseaux du cabinet d’histoire naturelle du jardin du roi. Vol. 2. Aillard & Constant-Chantpie, Paris, 251 pp. https://doi.org/10.5962/bhl.title.161301
- Vuilleumier, F. (1969) Pleistocene speciation in birds living in the high Andes. Nature, 223, 1179–1180. https://doi.org/10.1038/2231179a0
- Wallace, A.R. (1852) On the monkeys of the Amazon. Proceedings of the Zoological Society of London, 20, 107–110.
- Wallace, A.R. (1876) The Geographic Distribution of Animals. 2 Vols. Harper & Bros., New York, New York, 503 & 607 pp.
- Weir, J.T. (2009) Implications of genetic differentiation in Neotropical montane forest birds. Annals of the Missouri Botanical Gardens, 96, 410–433. https://doi.org/10.3417/2008011
- Weir, J.T., Faccio, M.S., Pulido-Santacruz, P., Barrera-Guzmán, A.O. & Aleixo, A. (2015) Hybridization in headwater regions, and the role of rivers as drivers of speciation in Amazonian birds. Evolution, 69, 1823–1834. https://doi.org/10.1111/evo.12696
- Weir, J.T. & Price, T.D. (2019) Song playbacks demonstrate slower evolution of song discrimination in birds from Amazonia than from temperate North America. PLoS Biology, 17 (10), e3000478. https://doi.org/10.1371/journal.pbio.3000478
- Willis, E.O. (1969) On the behavior of five species of Rhegmatorhina, ant-following antbirds of the Amazon basin. Wilson Bulletin, 81, 361–496.
- Winger, B.M. & Bates, J.M. (2015) The tempo of trait divergence in geographic isolation: Avian speciation across the Marañon Valley of Peru. Evolution, 69, 772–787. https://doi.org/10.1111/evo.12607
- Zimmer, J.T. (1931–1955) Studies of Peruvian birds, Nos. 1–66. American Museum Novitates, various volumes.
- Zimmer, J.T. (1932) Studies of Peruvian birds III. The genus Myrmotherula in Peru, with notes on extralimital forms. Part 1. American Museum Novitates, 523, 19 pp.
- Zimmer, K. & Isler, M.L. (2020a) Gray Antwren (Myrmotherula menetriesii). Version 1.0. In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (Eds.), Birds of the World. Cornell Lab of Ornithology, Ithaca, New York. [no pagination] https://doi.org/10.2173/bow.gryant1.01
- Zimmer, K. & Isler, M.L. (2020b) Long-winged Antwren (Myrmotherula longipennis). Version 1.0. In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (Eds.), Birds of the World. Cornell Lab of Ornithology, Ithaca, New York. [no pagination] https://doi.org/10.2173/bow.lowant1.01
- Zimmer, K. & Isler, M.L. (2020c) Plain-throated Antwren (Isleria hauxwelli). Version 1.0. In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (Eds.), Birds of the World. Cornell Lab of Ornithology, Ithaca, New York. [no pagination] https://doi.org/10.2173/bow.pltant1.01
- Zimmer, K. & Isler, M.L. (2020d) Rufous-bellied Antwren (Isleria guttata). Version 1.0. In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (Eds.), Birds of the World. Cornell Lab of Ornithology, Ithaca, New York. [no pagination] https://doi.org/10.2173/bow.rubant1.01