Abstract
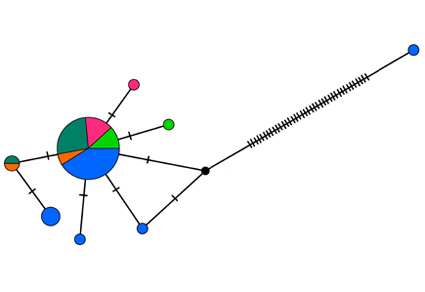
Hyptiasmus Kossack, 1911 is a genus of cyclocoelid digeneans infecting the body cavity, air sacs, and lungs of mainly anseriform and gruiform birds. Some species of this genus have extensive ranges, spanning more than one continent. Therefore, this taxon is potentially of interest for studying patterns of genetic diversity and the associated problems of taxonomic delimitation of species. We focused on the examination of Hyptiasmus specimens initially identified as Hyptiasmus oculeus Kossack, 1911. These digeneans were collected from the nasal sinuses of specimens of Eurasian Coot Fulica atra Linnaeus, 1758 taken in both European and Asian parts of Russia. Phylogenetic analyses based on 28S rRNA, nd1, and cytb markers, in combination with morphological data, indicate that the newly collected digeneans are in fact members of H. oculeus complex represented by true H. oculeus and a putative new species Hyptiasmus sp. The morphological differences between these species appear to be the length of the postovarian space and the position of the genital pore. A detailed morphological study revealed the presence of rudimentary ventral and oral suckers in gravid specimens of H. oculeus complex. We did not find any geographic structuredness of the haplotype diversity in true H. oculeus. Our findings do not support the recent proposal to resurrect H. singilum Witenberg, 1923 and H. brumpti Dollfus, 1948. In addition, we hypothesise that Paracyclocoelum Vainutis, Andreev & Voronova, 2024 is a synonym of Cyclocoelum Brandes, 1892.
References
- Assis, J.C.A. & Pinto, H.A. (2024) The Natural Infection of Freshwater Snails with the Avian Air Sac Fluke, Cyclocoelum mutabile (Trematoda: Cyclocoelidae), in Brazil. Diversity, 16 (7), 422. https://doi.org/10.3390/d16070422
- Assis, J.C.A., López-Hernández, D., Favoretto, S., Medeiros, L.B., Melo, A.L., Martins, N.R.S. & Pinto, H.A. (2021) Identification of the avian tracheal trematode Typhlocoelum cucumerinum (Trematoda: Cyclocoelidae) in a host-parasite-environment system: diagnosis, life cycle and molecular phylogeny. Parasitology, 148 (11), 1383–1391. https://doi.org/10.1017/S0031182021000986
- Blums, P.N. & Litzbarski, H. (1982) Coot, Fulica atra. In: Mihelsons, H.A. & Viksne, J.A. (Eds.), Migrations of Birds of Eastern Europe and Northern Asia: Falconiformis – Gruiformes. Nauka, Moscow, pp. 209–273.
- Borgarenko, L.F. (1984) Helminths of birds of Tajikistan. Book 2. Trematodes. Donish, Dushanbe, 210 pp. [in Russian]
- Brandes, G.P.H. (1892) Revision der Monostomiden. Centralblatt für Bakteriologie, 12 (15), 504–511.
- Bray, R.A., Cutmore, S.C. & Cribb, T.H. (2022) A paradigm for the recognition of cryptic trematode species in tropical Indo-west Pacific fishes: the problematic genus Preptetos (Trematoda: Lepocreadiidae) as a test-case. International Journal for Parasitology, 52 (2–3), 169–203. https://doi.org/10.1016/j.ijpara.2021.08.004
- Bykhovskaya-Pavlovskaya, I.E. (1949) Variability of morphological characters and its significance in the systematics of trematodes of the family Cyclocoelidae (Trematoda). Parazitologicheskii Sbornik, 11, 9–60.
- Bykhovskaya-Pavlovskaya, I.E. (1953) Fauna of trematodes of birds in west Siberia and its dynamics. Parazitologicheskii Sbornik, 15, 5–117.
- Chan, A.H.E., Chaisiri, K., Saralamba, S., Morand, S. & Thaenkham, U. (2021) Assessing the suitability of mitochondrial and nuclear DNA genetic markers for molecular systematics and species identification of helminthes. Parasites & Vectors, 14, 233. https://doi.org/10.1186/s13071-021-04737-y
- Chan, A.H.E., Thaenkham, U., Wichaita, T. & Saralamba, S. (2025) Validating a web application’s use of genetic distance to determine helminth species boundaries and aid in identification. BMC Bioinformatics, 26, 85. https://doi.org/10.1186/s12859-025-06098-0
- Diesing, C.M. (1850) Systema helminthum. Vol. I. Braumüller, Vindobonae, 679 pp.
- Dollfus, R.P. (1948) Sur deux monostomes (Cyclocoelidae) pourvus d’une ventrose ventral. Observations sur la classification des Cyclocoeloidea Albert Henry 1923, liste de leurs hotes, rȇpartition géographique. Annales de Parasitologie Humaine et Comparée, 23 (3–4), 129–199. https://doi.org/10.1051/parasite/1948233129
- Dronen, N.O. (2007) Revision of the family Cyclocoelidae Stossich, 1902 with the proposal of two new subfamilies and the description of a new species of Morishitium Witenberg, 1928 from the common snipe, Gallinago gallinago, from Texas, U.S.A. Zootaxa, 1563 (1), 55–68. https://doi.org/10.11646/zootaxa.1563.1.5
- Dronen, N.O. & Blend, C.K. (2007) Ophthalmophagus bucephali n. sp. (Digenea: Cyclocoelidae) from the American Goldeneye, Bucephala clangula americana (Anatidae), from the Central Flyway of North America and a Checklist of Goldeneye Parasites. Comparative Parasitology, 74 (1), 48–74. https://doi.org/10.1654/4221.1
- Dronen, N.O. & Blend, C.K. (2015) Updated keys to the genera in the subfamilies of Cyclocoelidae Stossich, 1902, including a reconsideration of species assignments, species keys and the proposal of a new genus in Szidatitreminae Dronen, 2007. Zootaxa, 4053 (1), 1–100. https://doi.org/10.11646/zootaxa.4053.1.1
- Dronen, N.O., Greiner, E.C., Ialeggio, D.M. & Nolan, T.J. (2009) Circumvitellatrema momota n. gen., n. sp. (Digenea: Cyclocoelidae: Cyclocoelinae) from a captive-hatched blue-crowned motmot, Momotus momota (Momotidae). Zootaxa, 2161 (1), 60–68. https://doi.org/10.11646/zootaxa.2161.1.5
- Dubois, G. (1959) Revision des Cyclocoelidae Kossack, 1911 (Trematoda). Revue Suisse de Zoologie, 66, 67–147. https://doi.org/10.5962/bhl.part.75207
- Dutton, H.R., Bullard, S.A. & Kelly, A.M. (2023) New Genus and Species of Cyclocoelidae Stossich, 1902 (Platyhelminthes: Digenea) Infecting the Nasopharyngeal Cavity of Canada Goose, Branta canadensis (Anseriformes: Anatidae) from Western Alabama. Journal of Parasitology, 109 (4), 349–356. https://doi.org/10.1645/23-10
- Edelenyi, B. (1974) Mételyek II.—Trematodes II. Közvetett fejlődéstí mételyek—Digenea. Akadémiai Kiadó, Budapest, 352 pp.
- Excoffier, L. & Lischer, H.E.L. (2010) Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10 (3), 564–567. https://doi.org/10.1111/j.1755-0998.2010.02847.x
- Feizullaev, N.A. (1969) The life-cycle of Hyptiasmus oculeus (Trematoda, Cyclocoelidae), parasite of nasal and orbital cavities of Fulica alra L. Parazitologiya, 3, 58–62.
- Feizullaev, N.A. (1980) Trematodes of the subfamily Cyclocoeloidea (morphology, biology, phylogeny and taxonomy). Elm, Baku, 209 pp.
- Galaktionov, K.V., Solovyeva, A.I. & Miroliubov, A. (2021) Elucidation of Himasthla leptosoma (Creplin, 1829) Dietz, 1909 (Digenea, Himasthlidae) life cycle with insights into species composition of the north Atlantic Himasthla associated with periwinkles Littorina spp. Parasitology Research, 120 (5), 1649–1668. https://doi.org/10.1007/s00436-021-07117-8
- Galosi, L., Heneberg, P., Rossi, G., Sitko, J., Magi, G.E. & Perrucci, S. (2019) Air sac trematodes: Morishitium polonicum as a newly identified cause of death in the common blackbird (Turdus merula). International Journal for Parasitology: Parasites and Wildlife, 9, 74–79. https://doi.org/10.1016/j.ijppaw.2019.03.021
- Ginetsinskaya, T.A. & Saakova, E.O. (1952) Migration of trematodes of the family Cyclocoelidae Koss. in the body of the final host. Doklady Akademii Nauk SSSR, 85, 1432–1436.
- Gomez-Puerta, L.A., Salas, M.Y., Lopez-Urbina, M.T. & Gonzalez, A.E. (2018) Diagnóstico morfológico y molecular de Cyclocoelum mutabile (Trematoda: Cyclocoelidae) en el Perú. Revista Peruana de Biología, 25 (3), 315–320. https://doi.org/10.15381/rpb.v25i3.15214
- Gu, Z. (2022) Complex heatmap visualization. iMeta, 1 (3), e43. https://doi.org/10.1002/imt2.43
- Gvozdev, E.V. (1962) Trematodes of game birds in South Kazakhstan. Trudy Instituta Zoologii, 16, 89–124.
- Heneberg, P. & Sitko, J. (2023) Morishitium polonicum (Machalska, 1980) is a junior synonym of Morishitium dollfusi (Timon-David, 1950) (Trematoda: Cyclocoeliidae). Parasitology Research, 122 (12), 3159–3168. https://doi.org/10.1007/s00436-023-08006-y
- Holterman, M., van der Wurff, A., van den Elsen, S., van Megen, H., Bongers, T., Holovachov, O., Bakker, J. & Helder, J. (2006) Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Molecular Biology and Evolution, 23 (9), 1792–1800. https://doi.org/10.1093/molbev/msl044
- Kanev, I., Radev, V. & Fried, B. (2002) Superfamily Cyclocoeloidea. In: Gibson, D.I., Jones, A. & Bray, R.A. (Eds.), Keys to the Trematoda. Vol. 1. CABI Publishing and The Natural History Museum, Wallingford and London, pp. 69–78. https://doi.org/10.1079/9780851995472.0127
- Kirillov, A.A., Kirillova, N.Yu., Shchenkov, S.V., Knyazev, A.E. & Vekhnik, V.A. (2024) The Morphological and molecular characterization of the avian trematodes Harrahium obscurum and Morishitium dollfusi (Digenea: Cyclocoelidae) from the Middle Volga Region (European Russia). Biology, 13 (8), 621. https://doi.org/10.3390/biology13080621
- Kossack, W.F.K. (1911) Ueber Monostomiden. Zoologische Jahrbücher. Abteilung Systematik, 31 (4), 491–590.
- Kostadinova, A., Herniou, E.A., Barrett, J. & Littlewood, D.T.J. (2003) Phylogenetic relationships of Echinostoma Rudolphi, 1809 (Digenea: Echinostomatidae) and related genera re-assessed via DNA and morphological analyses. Systematic Parasitology, 54 (3), 159–176. https://doi.org/10.1023/A:1022681123340
- Leigh, J.W. & Bryant, D. (2015) POPART: Full‐feature software for haplotype network construction. Methods in Ecology and Evolution, 6 (9), 1110–1116. https://doi.org/10.1111/2041-210X.12410
- Li, Y., Ma, X.X., Lv, Q.B., Hu, Y., Qiu, H.Y., Chang, Q.C. & Wang, C.R. (2020) Characterization of the complete mitochondrial genome sequence of Tracheophilus cymbius (Digenea), the first representative from the family Cyclocoelidae. Journal of Helminthology, 94, e101. https://doi.org/10.1017/S0022149X19000932
- Libert, C., Jouet, D., Ferté, H., Lemberger, K. & Keck, N. (2012) Air sac fluke Circumvitellatrema momota in a captive Blue-crowned motomot (Motomotus momota) in France. Journal of Zoo and Wildlife Medicine, 43 (3), 689–692. https://doi.org/10.1638/2012-0085.1
- Linnaeus, C. (1758) Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio Decima, Reformata. Salvius, Holmiæ, 824 pp. https://doi.org/10.5962/bhl.title.542
- Liu, S., Liu, Y., Chen, B., Lu, X., Jiang, D., Geng, L., Wang, X., Peng, K., Du, C., Ren, T. & Yang, X. (2023) The complete mitochondrial genome of Morishitium polonicum (Trematoda, Cyclocoelidae) and its phylogenetic implications. Parasitology Research, 122 (11), 2609–2620. https://doi.org/10.1007/s00436-023-07959-4
- Looss, A. (1902) Die Distomen-Unterfamilie der Haploporinae. Archives de Parasitologie, 6, 129–143.
- López-Jiménez, A., García-Varela, M. & Hernández-Orts, J.S. (2018) Review of five species of cyclocoelids (Digenea: Cyclocoelidae) from aquatic birds in Mexico with notes on their interspecific variation. Systematic Parasitology, 95 (8–9), 921–942. https://doi.org/10.1007/s11230-018-9825-x
- Machalska, J. (1980) Cyclocoelum polonicum sp. n. (Trematoda, Cyclocoelidae) from the thrushes Turdus philomelos Br. and T. merula L. Acta Parasitologica Polonica, 26 (14), 129–136.
- McLaughlin, J. & Marcogliese, D. (1983) The migration, growth and development of Cyclocoelum occuleum (Kossack, 1911) (Trematoda: Cyclocoelidae) in Fulica americana (Gm). Parasitology, 87 (2), 239–247. https://doi.org/10.1017/S0031182000052604
- Mehlis, E. (1831) Novae observationes de entozois. Auctore Dr. Fr. Chr. H. Creplin. Isis, Oken, 2, 166–199.
- Momeni, N., Rezaei, H.R., Ahmadpour, M. & Hapeman, P. (2022) Mitochondrial DNA cytochrome b diversity and phylogeny of the Eurasian coot (Fulica atra; Linnaeus, 1758) (Gruiformes: Rallidae). Journal of Wildlife and Biodiversity, 6 (2), 22–34.
- Morgan, J.A.T. & Blair, D. (1998) Relative merits of nuclear ribosomal internal transcribed spacers and mitochondrial CO1 and ND1 genes for distinguishing among Echinostoma species (Trematoda). Parasitology, 116 (3), 289–297. https://doi.org/10.1017/S0031182097002217
- Olson, P.D., Cribb, T.H., Tkach, V.V., Bray, R.A. & Littlewood, D.T.J. (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33 (7), 733–755. https://doi.org/10.1016/S0020-7519(03)00049-3
- Palm, V. (1963) Der Entwicklungszyklus Von Transcoelum oculеus (Kossack, 1911) Witenberg, 1923 (Fam. Cyclocoeliidae) Aus Dem Blesshuhn (Fulica atra L.). Zeitschrift für Parasitenkunde, 22 (6), 560–567. https://doi.org/10.1007/BF00259565
- Pavlov, A.V. (1962) Trematodes of rallid birds of the USSR. Trudy Gelminthologicheskoi Laboratorii AN SSSR, 12, 60–89. [in Russian]
- R Core Team (2022) R: A Language and environment for statistical computing. R foundation for statistical computing. Available from: http://www.r-project.org/index.html (accessed 21 June 2025)
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology, 61 (3), 539–542. https://doi.org/10.1093/sysbio/sys029
- Rozas, J., Ferrer-Mata, A., Sánchez-DelBarrio, J.C., Guirao-Rico, S., Librado, P., Ramos-Onsins, S.E. & Sánchez-Gracia, A. (2017) DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Molecular Biology and Evolution, 34 (12), 3299–3302. https://doi.org/10.1093/molbev/msx248
- Rudolphi, C.A. (1808) Entozoorum, sive vermium intestinalium historia naturalis (Vol. 1). Tabernae Librariae et Artium, Amstelaedami, 527 pp. https://doi.org/10.5962/bhl.title.14422
- Sey, O. (1966) On the helminth fauna of waterfowl (Fulica atra L.). Különleges Állatvilág Közlemények, 53 (1/4), 123–130.
- Sitko, J., Bizos, J. & Heneberg, P. (2017) Central European parasitic flatworms of the Cyclocoelidae Stossich, 1902 (Trematoda: Plagiorchiida): molecular and comparative morphological analysis suggests the reclassification of Cyclocoelum obscurum (Leidy, 1887) into the Harrahium Witenberg, 1926. Parasitology, 144 (4), 368–383. https://doi.org/10.1017/S0031182016001955
- Snyder, S.D. & Tkach, V.V. (2001) Phylogenetic and biogeographocal relationships among some Holoarctic Frog lung flukes (Digenea: Haematoloechidae). Journal of Parasitology, 87 (6), 1433–1440. https://doi.org/10.1645/0022-3395(2001)087[1433:PABRAS]2.0.CO;2
- Stossich, M. (1903) II Monostomum mutabile Zeder e le sue forme affini. Bolletino della Società Adriatica di Scienze Naturali in Trieste, 21, 1–40.
- Suleman, Khan, M.S., Heneberg, P., Zhou, C.-Y., Muhammad, N., Zhu, X.-Q. & Ma, J. (2019) Characterization of the complete mitochondrial genome of Uvitellina sp., representative of the family Cyclocoelidae and phylogenetic implications. Parasitology Research, 118 (7), 2203–2211. https://doi.org/10.1007/s00436-019-06358-y
- Suleman, Khan, M.S., Tkach, V.V., Muhammad, N., Zhang, D., Zhu, X.-Q. & Ma, J. (2020) Molecular phylogenetics and mitogenomics of three avian dicrocoeliids (Digenea: Dicrocoeliidae) and comparison with mammalian dicrocoeliids. Parasites & Vectors, 13 (1), 74. https://doi.org/10.1186/s13071-020-3940-7
- Szidat, L. & Szidat, U. (1966) Sobre mortandad masiva de gallaretas (Fulica leucoptera Vieillot) producida por Hyptiasmus oculeus Kossack, 1911 (Trematoda, Cyclocoelidae) observada en 1965, en el período de mínimo de las manchas solares. Comunicaciones del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Parasitología, 1, 17–30.
- Taft, S.J. (1972) Aspects of the Life History of Cyclocoelum oculeum (Trematoda: Cyclocoelidae). The Journal of Parasitology, 58 (5), 882. https://doi.org/10.2307/3286577
- Tamura, K., Stecher, G. & Kumar, S. (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution, 38 (7), 3022–3027. https://doi.org/10.1093/molbev/msab120
- Tatonova, Y.V. & Shumenko, P.G. (2021) Cytochrome b as a more promising marker for analysing the distribution vector for Metagonimus suifunensis (Trematoda: Heterophyidae). Parasitology, 148 (6), 760–766. https://doi.org/10.1017/S0031182021000275
- Timon-David, J. (1950) Un cyclocoelidé nouveau dans les sacs aériens de la pie, Cyclocoelum (Pseudhyptiasmus) dollfusi nov. sp. Bulletin de la Société Zoologique de France, 75, 243–246.
- Tkach, V., Pawlowski, J. & Mariaux, J. (2000) Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA. International Journal for Parasitology, 30 (1), 83–93. https://doi.org/10.1016/S0020-7519(99)00163-0
- Tkach, V.V., Kudlai, O. & Kostadinova, A. (2016) Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). International Journal for Parasitology, 46 (3), 171–185. https://doi.org/10.1016/j.ijpara.2015.11.001
- Trontelj, P. & Fišer, C. (2009) Perspectives: cryptic species diversity should not be trivialised. Systematics and Biodiversity, 7 (1), 1–3. https://doi.org/10.1017/S1477200008002909
- Urabe, M., Nor Hashim, N.E., Uni, S., Iwaki, T., Abdullah Halim, M.R., Marzuki, M.E., Mat Udin, A.S., Zainuri, N.A., Omar, H., Agatsuma, T., Uga, S., Takaoka, H., Azirun, M.S. & Ramli, R. (2020) Description and molecular characteristics of Morishitium polonicum malayense Urabe, Nor Hashim & Uni, n. subsp. (Trematoda: Cyclocoelidae) from the Asian glossy starling, Aplonis panayensis strigata (Passeriformes: Sturnidae) in Peninsular Malaysia. Parasitology International, 76, 102074. https://doi.org/10.1016/j.parint.2020.102074
- Utami, C.Y., Sholihah, A., Condamine, F.L., Thébaud, C. & Hubert, N. (2022) Cryptic diversity impacts model selection and macroevolutionary inferences in diversification analyses. Proceedings of the Royal Society B: Biological Sciences, 289 (1987), 20221335. https://doi.org/10.1098/rspb.2022.1335
- Vainutis, K.S., Voronova, A.N., Andreev, M.E. & Shchelkanov, M.Yu. (2024) New insights into the systematics of Cyclocoelidae (Trematoda: Echinostomatoidea) based on novel morphological and molecular data, with description of a new species and a new genus. Systematic Parasitology, 101 (6), 71. https://doi.org/10.1007/s11230-024-10192-x
- Vilas, R., Criscione, C.D. & Blouin, M.S. (2005) A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology, 131 (6), 839–846. https://doi.org/10.1017/S0031182005008437
- Witenberg, G.G. (1923) The trematodes of the family Cyclocoeliidae and a new principle of their systematics. Trudy gosudarstvennogo instituta eksperemental’noi veterinarii, 1 (1), 84–141.
- Witenberg, G.G. (1928) Notes on Cyclocoelidae. Annals & Magazine of Natural History, Series 10, 2 (11), 410–417. https://doi.org/10.1080/00222932808672903
- Yamaguti, S. (1971) Synopsis of digenetic trematodes of vertebrates. Vol. 1. Keigaku Publishing, Tokyo, 1074 pp.
- Zeder, J.G.H. (1800) Erster Nachtrag zur Naturgeschichte der Eingeweidewürmer von J. A. Li. Goeze, mit Zusätzen und Anmerkungen, herausgegeben von JGH Zeder. Mitteilungen, 6, 1–320.