Abstract
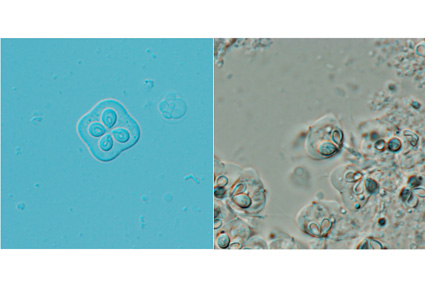
In this study, 20 specimens of Chaeturichthys stigmatias were collected from a fishing dock in Qingdao city, Shandong Province, China, and examined for parasitic infections. Kudoa spores were detected in the brains of 35% of the specimens. The mature spores were quadrate and hemispherical in lateral view, measuring 8.1±0.4 (7.1–8.8) μm in length, 13.5±0.4 (12.1–13.8) μm in width, and 11.8±0.4 (10.6–12.5) μm in thickness (n = 50). Each spore possessed four shell valves and four pyriform polar capsules measuring 4.0±0.2 (3.5–4.2) × 3.1±0.2 (2.7–3.5) μm. Morphological comparisons revealed distinct differences from previously described Kudoa species. Sequence analysis of the partial LSU rDNA further confirmed that this species did not match any known Kudoa species. Furthermore, the phylogenetic tree showed that it clusters with muscle-infecting Kudoa species but is distantly related to brain-infecting taxa. Based on morphological and molecular evidence, we describe Kudoa stigmatias sp. nov. as a novel species, representing the first documented case of myxosporean infection in Chaeturichthys species.
References
- Abdel-Baki, A.-A.S., Abdel-Haleem, H.M., Al-Quraishy, S., Azevedo, C. & Mansour, L. (2018) Ultrastructural and molecular characteristics of Kudoa crenimugilis n. sp. infecting intestinal smooth muscle of fringelip mullet Crenimugil crenilabis in the Red Sea. Diseases of aquatic organisms, 129, 53–62. https://doi.org/10.3354/dao03225
- Bartošová, P., Fiala, I. & Hypša, V. (2009) Concatenated SSU and LSU rDNA data confirm the main evolutionary trends within myxosporeans (Myxozoa: Myxosporea) and provide an effective tool for their molecular phylogenetics. Molecular phylogenetics and evolution, 53, 81–93. https://doi.org/10.1016/j.ympev.2009.05.018
- Cardim, J., Araújo-Neto, J., da Silva, D.T., Hamoy, I., Matos, E. & Abrunhosa, F. (2020) Kudoa yasai n. sp. (Multivalvulida: Kudoidae) from the skeletal muscle of Macrodon ancylodon (Sciaenidae) on the northern Atlantic coast, Brazil. Parasitology Research, 119, 1743–1752. https://doi.org/10.1007/s00436-020-06679-3
- Colunga-Ramírez, G., Aguirre-Macedo, M.L., Molnár, K., Székely, C., Sellyei, B. & Cech, G. (2025) Two new myxozoan parasites, Myxobolus mayarum n. sp. and Kudoa mayarum n. sp., infecting the Neotropical fish Mayan cichlid, Mayaheros urophthalmus (Günther, 1862) in the Yucatán Peninsula, Mexico. Acta Tropica, 262, 107527. https://doi.org/10.1016/j.actatropica.2025.107527
- Dyková, I., Buron, I.D., Fiala, I. & Roumillat, W.A. (2009) Kudoa inornata sp. n. (Myxosporea: Multivalvulida) from the skeletal muscles of Cynoscion nebulosus (Teleostei: Sciaenidae). Folia Parasitologica, 56, 91–8. https://doi.org/10.14411/fp.2009.014
- Eiras, J.C., Saraiva, A. & Cruz, C. (2014) Synopsis of the species of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida). Systematic Parasitology, 87, 153–180. https://doi.org/10.1007/s11230-013-9461-4
- Fiala, I. (2006) The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. International Journal for Parasitology, 36, 1521–1534. https://doi.org/10.1016/j.ijpara.2006.06.016
- Fiala, I., Bartošová-Sojková, P. & Whipps, C.M. (2015) Classification and phylogenetics of Myxozoa. In: Okamura, B., Gruhl, A. & Bartholomew, J. (Eds.), Myxozoan evolution, ecology and development. Springer, Cham, pp. 85–110. https://doi.org/10.1007/978-3-319-14753-6_5
- Kasai, A., Li, Y.C., Mafie, E. & Sato, H. (2016) New host records of monacanthid fish for three Kudoa spp. (K. septempunctata, K. thyrsites and K. shiomitsui) prevalent in the olive flounder (Paralichthys olivaceus), with the description of K. parathyrsites n. sp. from a black scraper (Thamnaconus modestus). Parasitology Research, 115, 2741–2755. https://doi.org/10.1007/s00436-016-5023-4
- Kasai, A., Tsuduki, H., Jimenez, L.A., Li, Y.C., Tanaka, S. & Sato, H. (2017) Incidence of three Kudoa spp., K. neothunni, K. hexapunctata and K. thunni (Myxosporea: Multivalvulida), in Thunnus tunas distributed in the western Pacific Ocean. Parasitology Research, 116, 1137–1150. https://doi.org/10.1007/s00436-016-5369-7
- Li, X.B., He, J., Ma, R.R., Sun, F.Y., Wu, W.X., Luo, H.M., Bai, L.H. & Qian, D. (2022) Morphological characterization and molecular phylogenetic analysis of Kudoa iwatai from large yellow croaker (Larimichthys crocea) as a new host in China. Animals, 12, 1145. https://doi.org/10.3390/ani12091145
- Li, Y.C., Inoue, K., Zhang, J.Y. & Sato, H. (2020a) Phylogenetic relationships of three Kudoa spp. with morphologically similar myxospores (K. iwatai, K. lutjanus and K. bora), with the redescription of K. uncinata and K. petala and description of a new species (K. fujitai n. sp.) in fishes in the South China Sea. Parasitology Research, 119, 1221–1236. https://doi.org/10.1007/s00436-020-06636-0
- Li, Y.C., Inoue, K., Tanaka, S., Zhang, J.Y. & Sato, H. (2020b) Identification of four new Kudoa spp. (Myxozoa: Myxosporea: Multivalvulida) in commercial fishes collected from South China Sea, Atlantic Ocean and Bering Sea by integrated taxonomic approach. Parasitology Research, 119, 2113–2128. https://doi.org/10.1007/s00436-020-06707-2
- Li, Y.C., Inoue, K., Zhang, J.Y. & Sato, H. (2024) Description of three new species of Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) in commercial marine fishes from southern China, and new host records. Folia Parasitologica, 71, 1–19. https://doi.org/10.14411/fp.2024.018
- Liu, X., Zhang, C., Ren, Y. & Xu, B. (2015) Spatiotemporal variation in the distribution and abundance of Chaeturichthys stigmatias in the Yellow River estuary and adjacent waters. Journal of Fishery Sciences of China, 22, 791–798. https://doi.org/10.3724/SP.J.1118.2015.140426
- Lom, J. & Arthur, J. (1989) A guideline for the preparation of species descriptions in Myxosporea. Journal of Fish Diseases, 12, 151–156. https://doi.org/10.1111/j.1365-2761.1989.tb00287.x
- Lom, J. & Dyková, I. (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitologica, 53, 1–36. https://doi.org/10.14411/fp.2006.001
- Mansour, L., Harrath, A.H., Abdel-Baki, A.A.S., Alwasel, S., Al-Quraishy, S. & Al Omar, S.Y. (2015) Kudoa saudiensis sp. n. (Myxosporea: Multivalvulida) infecting oocytes of the Indian mackerel Rastrelliger kanagurta (Perciformes: Scombridae). Folia Parasitologica, 62 (1), 10. https://doi.org/10.14411/fp.2015.010
- Meng, K., Wang, J., Zhang, C., Ren, Y. & Xu, B. (2017) The fishery biological characteristics of Chaeturichthys stigmatias in the Yellow River estuary and its adjacent waters. Journal of Fishery Sciences of China, 24, 939–945. https://doi.org/10.3724/SP.J.1118.2017.17083
- Moran, J.D.W., Whitaker, D.J. & Kent, M.L. (1999) A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture, 172, 163–196. https://doi.org/10.1016/S0044-8486(98)00437-2
- Ohnishi, T., Obara, T., Arai, S., Yoshinari, T. & Sugita-Konishi, Y. (2018) Quantitative analysis of Unicapsula seriolae in greater amberjack associated with unidentified food-borne disease. Journal of the Food Hygienic Society of Japan, 59, 24–29. https://doi.org/10.3358/shokueishi.59.24
- Posada, D. (2008) jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25, 1253–1256. https://doi.org/10.1093/molbev/msn083
- Ren, Y. (2022) Fishery Resources and Habitat Environment in Haizhou Bay. China Agriculture Press, Beijing, 187 pp.
- Sakai, H., Kawai, T., Zhang, J. & Sato, H. (2019) New host records of three Kudoa spp. (K. yasunagai, K. thalassomi and K. igami) with notable variation in the number of shell valves and polar capsules in spores. Parasitology Research, 118, 143–157. https://doi.org/10.1007/s00436-018-6144-8
- Shirakashi, S., Morita, A., Ishimaru, K. & Miyashita, S. (2012) Infection dynamics of Kudoa yasunagai (Myxozoa: Multivalvulida) infecting brain of cultured yellowtail Seriola quinqueradiata in Japan. Diseases of aquatic organisms, 101, 123–130. https://doi.org/10.3354/dao02513
- Sun, Y., Wei, T. & Jin, X. (2015) Unusual features of control region and a novel NADH 6 genes in mitochondrial genome of the finespot goby, Chaeturichthys stigmatias (Perciformes, Gobiidae). Mitochondrial DNA, 26, 665–667. https://doi.org/10.3109/19401736.2013.840598
- Suzuki, J., Murata, R., Yokoyama, H., Sadamasu, K. & Kai, A. (2015) Detection rate of diarrhoea-causing Kudoa hexapunctata in Pacific bluefin tuna Thunnus orientalis from Japanese waters. International Journal of Food Microbiology, 194, 1–6. https://doi.org/10.1016/j.ijfoodmicro.2014.11.001
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. https://doi.org/10.1093/molbev/mst197
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. & Higgins, D.G. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic acids research, 25, 4876–4882. https://doi.org/10.1093/nar/25.24.4876
- Velasco, M., Eduard, J., Neto, J.L.S., Dias, L.d.N.S., Matos, E. & Gonçalves, E.C. (2022) Kudoa rousseauxii n. sp.(Cnidaria: Multivalvulida) infects the skeletal muscles of the freshwater fish Brachyplatystoma rousseauxii in the Amazon River. Acta Parasitologica, 67, 962–969. https://doi.org/10.1007/s11686-022-00539-z
- Wang, H.W., Zhu, N., Cai, D.B., Wang, Y.F., Liu, Y.W., Tian, S.Q., Wang, Y. & Huo, R. (2014) Evaluation and content of various heavy metals found in the fish (Chaeturichthys stigmatias Richardson) collected from the oil spill area of Bohai Bay (China) during the Summer. Advanced Materials Research, 955, 1448–1451. https://doi.org/10.4028/www.scientific.net/AMR.955-959.1448
- Whipps, C., Grossel, G., Adlard, R., Yokoyama, H., Bryant, M., Munday, B. & Kent, M. (2004) Phylogeny of the Multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. Journal of Parasitology, 90, 618–622. https://doi.org/10.1645/GE-153R
- Whipps, C.M. & Kent, M.L. (2006) Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). Journal of Eukaryotic Microbiology, 53, 364–373. https://doi.org/10.1111/j.1550-7408.2006.00114.x
- Wu, H.L. & Zhong, J.S. (2008) n.k. In: Fauna Sinica, Osteichthyes, Perciformes. Science Press, Beijing, pp. 311–312.
- Xu, L., Zhao, X., Huang, Y., Xin, Z. & Zhang, J. (2025) Morphological and molecular characterization of Myxobolus aculeatus n. sp. (Myxozoa: Myxosporea) from the ovary of Macrognathus aculeatus, Bloch, 1786 (Synbranchiformes: Mastacembelidae) in China. Parasitology International, 106, 103039. https://doi.org/10.1016/j.parint.2025.103039
- Yu, X., Cao, L., Liu, J., Zhao, B., Shan, X. & Dou, S. (2014) Application of otolith shape analysis for stock discrimination and species identification of five goby species (Perciformes: Gobiidae) in the northern Chinese coastal waters. Journal of Oceanology and Limnology, 32, 1060–1073. https://doi.org/10.1007/s00343-015-4022-0
- Zhang, D., Gao, F., Jakovlić, I., Zou, H., Zhang, J., Li, W.X. & Wang, G.T. (2020) PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20, 348–355. https://doi.org/10.1111/1755-0998.13096