Abstract
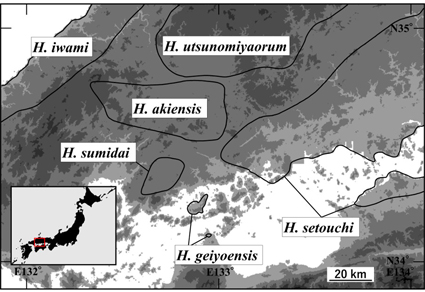
Hynobius akiensis sensu lato has recently been split into three species based on short sequence analyses of cyt-b gene of mtDNA and without data of nuclear DNA, and strange sympatric distribution in some areas has been indicated in two species. We analyzed nuclear DNA marker (SNPs) and complete sequence of cyt-b in H. akiensis sensu lato to reassess species delimitation and genetic introgression among species. As a result, we found two lineages with discordant mitochondrial and nuclear DNA in some areas. Of H. akiensis sensu lato, each of the two contains the type locality of two species recently reported (H. sumidai and H. geiyoensis), and the use of these names has been previously advocated. However, their sympatric distribution was rejected based on nuclear DNA data, which we consider is more reliable than mtDNA. We thus clarify geographic boundary of these two species and revise the species delimitations.
References
- Akiyoshi, H. & Matsuno, A. (2014) Hynobius nebulosus. In: Shimane Red Data Book Revision Committee (Ed.), Revised Shimane Red Data Book, 2014, Animals, pp. 73.
- Aoki, G., Matsui, M. & Nishikawa, K. (2013) Mitochondrial cytochrome b phylogeny and historical biogeography of the Tohoku salamander, Hynobius lichenatus (Amphibia, Caudata). Zoological Science, 30, 167–173. https://doi.org/10.2108/zsj.30.167
- Dufresnes, C. & Jablonski, D. (2022) A genomics revolution in amphibian taxonomy. Science, 377, 1272. https://doi.org/10.1126/science.ade5002
- Eaton, D.A.R. (2020) ipyrad: Interactive assembly and analysis of RADseq datasets. Bioinformatics, 36, 2592–2594. https://doi.org/10.1093/bioinformatics/btz966
- Eto, K., Matsui, M. & Sugahara, T. (2013) Discordance between Mitochondrial DNA Genealogy and Nuclear DNA Genetic Structure in the Two Morphotypes of Rana tagoi tagoi (Amphibia: Anura: Ranidae) in the Kinki Region, Japan. Zoological Science, 30, 553–558. https://doi.org/10.2108/zsj.30.553
- Felsenstein, J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
- Frost, D. (2022) Amphibian species of the world. https://amphibiansoftheworld.amnh.org/ (accessed 7 August 2022)
- Hammer, Ø., Harper, D.A.T. & Ryan, P.D. (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
- Huelsenbeck, J.P. & Hillis, D.M. (1993) Success of phylogenetic methods in the four-taxon case. Systematic Biology, 42, 247–264. https://doi.org/10.1093/sysbio/42.3.247
- Huelsenbeck, J.P., Ronquist, F., Nielsen, R. & Bollback, J.P. (2001) Bayesian inference of phylogeny and its impact on evolutionary biology. Science, 294, 2310–2314. https://doi.org/10.1126/science.1065889
- Itano, K., Fujiwara, Y., Ikeuchi, K., Kobayashi, S. & Omori, K. (2016) Study on the breeding ecology of Clouded salamander (Hynobius nebulosus) in Imabari, Ehime Prefecture. Bulletin of the Ehime Pref. Museum, 21, 1–18.
- Jakobsson, M. & Rosenberg, N.A. (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics, 23, 1801–1806. https://doi.org/10.1093/bioinformatics/btm233
- Japan Meteorological Agency (2022) Historical Weather Data Search. Available from: https://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed 15 October 2022)
- Kusano, T. & Hayase, N. (1990) Geographic variation in egg size in H. tokyoensis. Bulletin of the Population Ecology Society, 46, 5–12.
- Leaché, A.D. & Reeder, T.W. (2002) Molecular systematics of the eastern fence lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Systematic Biology, 51, 44–68. https://doi.org/10.1080/106351502753475871
- Matsui, M., Yoshikawa, N., Tominaga, A., Sato, T., Takenaka, S., Tanabe, S., Nishikawa, K. & Nakabayash, S. (2008) Phylogenetic relationships of two Salamandrella species as revealed by mitochondrial DNA and allozyme variation (Amphibia: Caudata: Hynobiidae). Molecular Phylogenetics and Evolution, 48, 84–93. https://doi.org/10.1016/j.ympev.2008.04.010
- Matsui, M., Okawa, H., Nishikawa, K., Aoki, G., Eto, K., Yoshikawa, N., Tanabe, S., Misawa, Y. & Tominaga, A. (2019) Systematics of the widely distributed Japanese clouded salamander, Hynobius nebulosus (Amphibia: Caudata: Hynobiidae), and its closest relatives. Current Herpetology, 38, 32–90. https://doi.org/10.5358/hsj.38.32
- Ministry of the Environment (2020) Publication of the Ministry of the Environment's Red List 2020. Available from: https://www.env.go.jp/press/107905.html (accessed 24 April 2023)
- Misawa, Y. (1989) The method of counting costal grooves. In: Matsui, M., Hikida, T. & Goris, R. C. (Eds.), Current Herpetology in East Asia, Herpetological Society of Japan, Kyoto, pp. 129–134.
- Nishikawa, K., Matsui, M., Tanabe, S. & Sato, S. (2007) Morphological and allozymic variation in Hynobius boulengeri and H. stejnegeri (Amphibia: Urodela: Hynobiidae). Zoological Science, 24, 752–766. https://doi.org/10.2108/zsj.24.752
- Okawa, H. (2021) H. akiensis. In: Rare Species Subcommittee (Ed.), Red Data Book Hiroshima, 2021, pp.142.
- Okawa, H. & Utsunomiya, T. (1989) Hynobius nebulosus from Hiroshima Prefecture. In: Matsui, M., Hikida, T. & Goris, R.C. (Eds.), Current Herpetology in East Asia. Herpetological Society of Japan, Kyoto, pp. 142–146.
- Okawa, H. & Utsunomiya, T. (1992) On the toe bones of Hynobius nebulosus. Japanese Journal of Herpetology, 14, 214.
- Okawa, H., Okuno, T. & Utsunomiya, T. (2019) Major groups of Hynobius nebulosus in Western Japan. Bulletin of the Herpetological Society of Japan, 2019, 9–21.
- Okawa, H., Okuno, T. & Utsunomiya, T. (2009) Variation in Hynobius nebulosus from Western Japan. Bulletin of the Herpetological Society of Japan, 2009, 6–12.
- Okawa, H., Utsunomiya, T. & Okuno, T. (1999) Four toes population of Hynobius nebulosus. Hibakagaku (Journal of the Hiba Society of Natural History), 191, 4.
- Okawa, H., Okuno, T. & Utsunomiya, T. (2005) A group of Hynobius nebulosus distributed in Abu, Tsuwano and Yamaguchi region. Amphibian History, 14, 11–14.
- Okawa, H., Okuno, T. & Utsunomiya, T. (2007) Major groups of Hynobius nebulosus in Western Japan. Bulletin of the Herpetological Society of Japan, 2007, 58–59.
- Pritchard, J.K., Stephens, M. & Donnelly, P. (2000) Inference of population structure using multilocus genotype data. Genetics, 155, 945–959. https://doi.org/10.1093/genetics/155.2.945
- Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. (2014) Tracer. Version 1.6. Institute of Evolutionary Biology, University of Edinburgh. Available from: http://tree.bio.ed.ac.uk/software/tracer/ (accessed 7 August 2022)
- Ronquist, F., Teslenko, M., Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Sato, I. (1943) A Monograph of the Tailed Batrachians of Japan. Nippon Shuppan-sha, Osaka, 520 pp.
- Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Sugawara, H., Iwata, T., Yamashita, H. & Nagano, M. (2021) Taxonomic Reassessment of the Izumo Lineage of Hynobius utsunomiyaorum: Description of a New Species from Chugoku, Japan. Animals, 11, 2187. https://doi.org/10.3390/ani11082187
- Sugawara, H., Naito, J., Iwata, T. & Nagano, M. (2022) Molecular Phylogenetic and Morphological Problems of the Aki Salamander Hynobius akiensis: Description of Two New Species from Chugoku, Japan. Bulletin of The Kanagawa Prefectural Museum Natural Science, 51, 35–46. https://doi.org/10.32225/bkpmnh.2022.51_35
- Suyama, Y. & Matsuki, Y. (2015) MIG-seq: an effective PCR-based method for genome-wide singlenucleotide polymorphism genotyping using the next-generation sequencing platform. Scientific Reports, 5, 16963. https://doi.org/10.1038/srep16963
- Tanabe, A.S. (2011) Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Molecular Ecology Resources, 11, 914–921. https://doi.org/10.1111/j.1755-0998.2011.03021.x
- Tanabe, S. & Okayama, K. (2001) About the lentic salamander discovered in Ehime Prefecture, Japan. Bulletin of the Ehime Prefecture Museum, 15, 23–27.
- Tanabe, S. (2014) H. nebulosus. In: Ehime Red Data Book Revision Committee (Ed.), Red Data Book Ehime, 2014, pp. 97.
- Tominaga, A., Matsui, M. & Nishikawa, K. (2019a) Two new species of lotic breeding salamanders (Amphibia, Caudata, Hynobiidae) from western Japan. Zootaxa, 4550 (4), 525–544. https://doi.org/10.11646/zootaxa.4550.4.3
- Tominaga, A., Matsui, M., Tanabe, S. & Nishikawa, K. (2019b) A revision of Hynobius stejnegeri, a lotic breeding salamander from western Japan, with a description of three new species (Amphibia, Caudata, Hynobiidae). Zootaxa, 4651 (3), 401–433. https://doi.org/10.11646/zootaxa.4651.3.1
- Tsurusaki, N. (2007) The Chugoku Mountains a hotspot of geographical differentiation of species. Taxa, 22, 3–14. https://doi.org/10.19004/taxa.22.0_3
- Utsunomiya, T., Utsunomiya, Y., Okawa, H. & Naito, J. (1996) Amphibians from the area around Haizuka projected reservoir in Hiroshima Prefecture, Southwest Japan. Reprinted from Natural History of Haizuka, 1996, 177–215.
- Vázquez, C.F., Lourenço, A., Antón, G.V. (2021) Riverine barriers to gene frow in a salamander with both aquatic and terrestrial reproduction. Evolutionary Ecology, 35, 483–511. https://doi.org/10.1007/s10682-021-10114-z
- Zharkikh, A. (1994) Estimation of evolutionary distances between nucleotide sequences. Journal of Molecular Evolution, 39, 315–329. https://doi.org/10.1007/BF00160155