Abstract
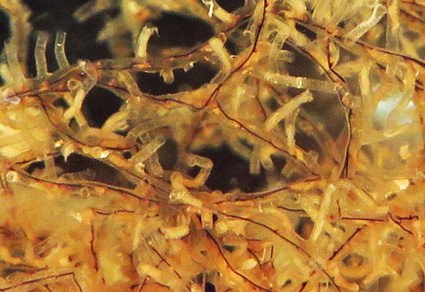
All eight extant species of Rhabdopleura described between 1869 and 2018 are provisionally accepted as valid based on a review of the literature and new data on two little-known species from the Azores. Additionally, four new species are described from the New Zealand region, increasing global diversity by 50%, and a dichotomous key to all 12 described species is provided based on morphological criteria. The distinction between colony morphologies based on erect-tube inception is regarded as particularly helpful in initial characterization of species. Erect ringed tubes are either produced directly from the surface of creeping-tubes or indirectly, i.e. a short adherent side branch from a creeping tube is interpolated between the creeping tube and an erect tube; such side branches are blind-ending. These two modes of erect-tube origination are here respectively termed direct and indirect. Species with indirect erect-tube budding are predominant in the North Atlantic whereas species with direct erect-tube budding dominate in New Zealand waters. The only indirect-erect species from New Zealand, Rhabdopleura chathamica n. sp., was discovered on deepwater coral from 1008‒1075 m, constituting the deepest record of the genus to date. Rhabdopleura emancipata n. sp., collected only in a detached state, constitutes a three-dimensional tangled growth that grew freely into the water column—a unique morphology hitherto unknown among extant species. Owing to this growth mode, it provided a substratum for epibionts from several phyla. Rhabdopleura francesca n. sp. and Rhabdopleura decipula n. sp. are morphologically very similar but are distinguishable by their distinct placements in a phylogeny based on 16S mitochondrial and 18S nuclear rRNA genes. Phylogenetic reconstructions based on rRNA and mitochondrial genome data contribute to an updated phylogeny of all Rhabdopleura species sequenced thus far, some of which require more molecular sequences and morphological analyses for taxonomic determination.
References
- Allman, G.J. (1869) On Rhabdopleura, a new form of Polyzoa, from deep-sea dredging in Shetland. Quarterly Journal of Microscopical Science, New Series, 4, 95‒109, pl. 8.
- Andres, D. (1977) Graptolithen aus ordovizischen Geschieben und die frühe Stammesgeschichte der Graptolithen. Paläontologische Zeitschrift, 51, 52‒93. https://doi.org/10.1007/BF02986602
- Bankevich, A., Nurk, S., Antipov, D., Gurevich, A.A., Dvorkin, M., Kulikov, A.S., Lesin, V.M., Nikolenko, S.I., Pham, S., Prjibelski, A.D., Pyshkin, A.V., Sirotkin, A.V., Vyahhi, N., Tesler, G., Alekseyev, M.A. & Pevzner, P.A. (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology, 19, 455–477. https://doi.org/10.1089/cmb.2012.0021
- Barnes, R.D. (1977) New record of a pterobranch hemichordate from the Western Hemisphere. Bulletin of Marine Science, 27, 340‒343.
- Bateson, W. (1885) The later stages in the development of Balanoglossus Kowalevskii, with a suggestion as to the affinities of the Enteropneusta. Quarterly Journal of Microscopical Science, New Series, 25 (Supplement), 81‒122, pls. 4‒9. https://doi.org/10.1242/jcs.S2-25.S1.81
- Beli, E., Aglieri, G., Strano, F., Maggioni, D., Telford, M., Piraino, S. & Cameron, C.B. (2018) The zoogeography of extant rhabdopleurid hemichordates (Pterobranchia: Graptolithina), with a new species from the Mediterranean Sea. Invertebrate Systematics, 32, 100‒110. https://doi.org/10.1071/IS17021
- Bronn, H.G. (1849) Handbuch einer Geschichte der Natur. 3,2: III. Theil: Organisches Leben (Schluß). Index palaeontologicus oder Uebersicht der bis jetzt bekannten fossilen Organismen. B, Enumerator palaeontologicus : Systematische Zusammenstellung und geologische Entwickelungs-Gesetze der organischen Reiche. Schweizerbart, Stuttgart, 1106 pp. https://doi.org/10.5962/bhl.title.102095
- Bulman, O.M.B. (1970) Graptolithina. In: Teichert, C. (ed.), Treatise on Invertebrate Paleontology, Part V, second edition. Geological Society of America and University of Kansas Press, Lawrence, Kansas, xxx + 163 p.
- Burdon-Jones, C. (1854) The habitat and distribution of Rhabdopleura normani Allman. Universitetet i Bergen Årbok, Naturvitenskapelig Rekke, 11, 1‒17.
- Cannon, J.T., Rychel, A.L., Eccleston, H. Halanych, K.M. & Swalla, B.J. (2009) Molecular phylogeny of Hemichordata, with updated status of deep-sea enteropneusts. Molecular Phylogenetics and Evolution, 52, 17‒24. https://doi.org/10.1016/j.ympev.2009.03.027
- Cannon, J.T., Kocot, K.M., Waits, D.S., Weese, D.A., Swalla, B.J., Santos, S.R. & Halanych, K.M. (2014) Phyogenomic resolution of the hemichordate and echinoderm clade. Current Biology, 24, 2827‒2832. https://doi.org/10.1016/j.cub.2014.10.016
- Capella-Gutiérrez, S., Silla-Martínez, J.M. & Gabaldón, T. (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics, 25, 1972–1972. https://doi.org/10.1093/bioinformatics/btp348
- Carter, H.J. (1880) Report on specimens dredged up from the Gulf of Manaar and presented to the Liverpool Free Museum by Capt. W. H. Cawne Warren. Annals and Magazine of Natural History, Series 5, 6, 35‒61, pls. 4‒6. https://doi.org/10.1080/00222938009458893
- Chapman, A.J., Durman, P.N. & Rickards, R.B. (1995) Rhabdopleuran hemichordates: new fossil forms and review. Proceedings of the Geological Association, 106, 293–303. https://doi.org/10.1016/S0016-7878(08)80240-4.
- Chen, S., Zhou, Y., Chen, Y. & Gu, J. (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics, 34, i884–i890. https://doi.org/10.1093/bioinformatics/bty560
- Darriba, D., Posada, D., Kozlov, A.M., Stamatakis, A., Morel, B. & Flouri, T. (2020) ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution, 37, 291‒294. https://doi.org/10.1093/molbev/msz189
- Dilly, P.N. (1973) The larva of Rhabdopleura compacta (Hemichordata). Marine Biology, 18, 69‒86. https://doi.org/10.1007/bf00347923
- Dilly, P.N. (1985a) The prosicular stage of Rhabdopleura (Pterobranchia: Hemichordata). Journal of Zoology, London, A, 206, 163‒174. https://doi.org/10.1111/j.1469-7998.1985.tb05642.x
- Dilly, P.N. (1985b) A note on the status of Rhabdopleura from Bermuda. Journal of the Marine Biological Association of the United Kingdom, 65, 987‒991. https://doi.org/10.1017/S0025315400019469
- Dilly, P.N. & Ryland, J.S. (1985) An intertidal Rhabdopleura (Hemichordata, Pterobranchia) from Fiji. Journal of Zoology, London, A, 205, 611‒623. https://doi.org/10.1111/j.1469-7998.1985.tb03548.x
- Donath, A., Jühling, F., Al-Arab, M., Bernhart, S.H., Reinhardt, F., Stadler, P.F., Middendorf, M. & Bernt, M. (2019) Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Research, 47, 10543–10552. https://doi.org/10.1093/nar/gkz833
- Fowler, G.H. (1892) The morphology of Rhabdopleura Normani Allm. In: Festschrift zum siebenzigsetn Geburtstage Rudolph Leukarts dem verehrten Jubilar dargebracht von seinen dankbaren Schülern. Verlag Wilhelm Engelmann, Leipzig, pp. 293‒297, pl. 30.
- Gordon, D.P. (1993) Bryozoa: The ascophorine infraorders Cribriomorpha, Hippothoomorpha and Umbonulomorpha mainly from New Caledonian waters. In: Crosnier, A. (Ed.), Résultats des Campagnes MUSORSTOM. Vol. 11. Mémoires du Muséum National d’Histoire Naturelle, 158, 299347.
- Gordon, D.P. (2013) Life on the edge: Parachnoidea (Ctenostomata) and Barentsia (Kamptozoa) on bathymodiolin mussels from an active submarine volcano in the Kermadec volcanic arc. In: Ernst, A., Schäfer, P. & Scholz, J. (Eds.), Bryozoan Studies 2010. Lecture Notes in Earth System Sciences, 143, pp. 75–88. https://doi.org/10.1007/978-3-642-16411-8_6
- Gordon, D.P., Quek, Z.B.R., Orr, R.J.S., Waeschenbach, A., Huang, D., Strano, F., Ramsfjell, M.H. & Liow, L.H. (2023) Morphological diversity and a ribosomal phylogeny of Rhabdopleura (Hemichordata: Graptolithina) from the Western Pacific (Singapore and New Zealand), with implications for a re-evaluation of rhabdopleurid species diversity. Marine Biodiversity, 53(4), 1‒18. https://doi.org/19.1007/s12526-022-01310-3
- Halanych, K.M., Lutz, R.A. & Vrijenhoek, R.C. (1998) Evolutionary origins and age of vestimentiferan tube-worms. Cahiers de Biologie Marine, 39, 355–358.
- Halanych, K.M., Tassia, M.G. & Cannon, J.T. (2018) Pterobranchia. In: Schmidt-Rhaesa, A. (ed.), Handbook of Zoology: Miscellaneous Invertebrates. De Gruyter, Berlin, pp. 267‒282. https://doi.org/10.1515/9783110489279-008
- Harmer, S.F. (1905) The Pterobranchia of the Siboga Expedition with an account of other species. Siboga-Expeditie, 26, 1‒133. https://doi.org/10.5962/bhl.title.11734
- Hayward, P.J. (1981) The Cheilostomata (Bryozoa) of the deep-sea. Galathea Report, 15, 21‒68, pl. 5.
- Hillis, D.M. & Dixon, M.T. (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Quarterly Review of Biology, 66, 411‒453. https://doi.org/10.1086/417338
- Hincks, T. (1880) A History of the British Marine Polyzoa. Vols. 1 & 2. Van Voorst, London, cxli + 601 pp. & 83 pls.
- Hopkinson, J. & Lapworth, C. (1875) Descriptions of the graptolites of the Arenig and Llandeilo rocks of St. David’s. Quarterly Journal of the Geological Society of London, 31, 631‒672, pls. 33‒37. https://doi.org/10.1144/gsl.jgs.1875.031.01-04.49
- Johnston, T.H. (1937) A note on the occurrence of Rhabdopleura annulata in South Australian waters. Records of the South Australian Museum, 6, 105‒107.
- Jullien, J. (1890) Description d’un Bryozoaire nouveau du genre Rhabdopleura. Bulletin de la Société Zoologique de France, 15, 180–183. https://doi.org/10.5962/bhl.part.18723
- Jullien, J. & Calvet, L. (1903) Bryozoaires provenant des campagnes de l’Hirondelle. Résultats des Campagnes scientifiques accompli par le Prince Albert I, 23, 1‒188, 18 pls.
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30, 772‒780. https://doi.org/10.1093/molbev/mst010
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, M., Buxton, S. Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P. & Drummond, A. (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647‒1649. https://doi.org/10.1093/bioinformatics/bts199
- Kluge, H. (1948) On the occurrence of Rhabdopleura in the Barents Sea. Trudy Murmanskaya Biologicheskaya Stantsiya, 1, 276‒283. [in Russian]
- Kozlov, A.M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. (2019) RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics, 35, 4453–4455. https://doi.org/10.1093/bioinformatics/btz305
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Lankester, E.R. (1877) Notes on the embryology and classification of the animal kingdom: comprising a revision of speculations relative to the origin and significance of the germ-layers. Quarterly Journal of Microscopical Science, New Series, 17, 399‒454, pl. 25. https://doi.org/10.1242/jcs.s2-17.68.399
- Lankester, E.R. (1884) A contribution to the knowledge of Rhabdopleura. Quarterly Journal of Microscopical Science, 24, 622–647, pls. 37–41. https://doi.org/10.1242/jcs.s2-24.96.622
- Lester, S.M. (1988) Settlement and metamorphosis of Rhabdopleura normani (Hemichordata: Pterobranchia). Acta Zoologica, 69, 111‒120. https://doi.org/10.1111/j.1463-6395.1988.tb00907.x
- Li, Y., Kocot, K.M., Tassia, M.G., Cannon, J.T., Bernt, M., Halanych, K.M. (2019) Mitogenomics reveals a novel genetic code in Hemichordata. Genome Biology and Evolution, 11, 29–40. https://doi.org/10.1093/gbe/evy254
- López Gappa, J.J. (1987) Presencia del género Rhabdopleura (Hemichordata), Pterobranchia) en la plataforma continental, Argentina. Physis A, 45 (108), 33–36.
- Maletz, J. (2014) The classification of the Pterobranchia (Cephalodiscida and Graptolithina). Bulletin of Geosciences, 89, 477‒540. https://doi.org/10.3140/bull.geosci.1465
- Maletz, J. (2015) Graptolite reconstructions and interpretations. Paläontologische Zeitschrift, 89, 271‒286. https://doi.org/10.1007/s12542-014-0234-4
- Maletz, J. & Beli, E. (2018) Part V, Second Revision, Chapter 15: Subclass Graptolithina and incertae sedis family Rhabdopleuridae: Introduction and systematic descriptions. Treatise Online, 101, 1‒14. https://doi.org/10.17161/to.v0i0.7053
- Maletz, J., Lenz, A.C. & Bates, D.E.B. (2016) Part V, Second Revision, Chapter 4: Morphology of the pterobranch tubarium. Treatise Online, 76, 1‒63. https://doi.org/10.17161/to.v0i0.5727
- Maletz, J., Bates, D.E.B., Brussa, E.D., Cooper, R.A., Lenz, A.C., Riva, J.F., Toro, B.A. & Zhang, Y. (2014) Part V, Revision 2, Chapter 12: Glossary of the Hemichordata. Treatise Online, 62, 1‒23. https://doi.org/10.17161/to.v0i0.4710
- Mierzejewski, P. (1986) The enigma of graptolite ancestry: lesson from a phylogenetic debate. In: Hoffman, A. & Nitecki, M. (Eds.), Problematic Fossil Taxa. Oxford University Press, New York, New York, pp. 184–225.
- Mierzejewski, P. & Kulik C (2001) Graptolite-like fibril pattern in the fusellar tissue of Palaeozoic rhabdopleurid pterobranchs. Acta Palaeontologica Polonica, 46, 349–366.
- Mitchell, C.E., Melchin, M.J., Cameron, C.B. & Maletz, J. (2013) Phylogenetic analysis reveals that Rhabdopleura is an extant graptolite. Lethaia, 46, 34‒56. https://doi.org/10.1111/j.1502-3931.2012.00319.x
- Nicholson, H.A. (1872) A Monograph of the British Graptolitidae. Blackwood & Sons, Edinburgh & London, x + 133 pp.
- Norman, A.M. (1869) Shetland final dredging report, Pt. II. On the Crustacea, Tunicata, Polyzoa, Echinodermata, Actinozoa, Hydrozoa and Porifera. Report of the British Association for the Advancement of Science for 1868, 1869, 247‒336.
- Norman, J.R. (1921) Rhabdopleura. British Antarctic “Terra Nova” Expedition Natural History Reports, Zoology, 4, 95‒102.
- Palumbi, S.R. (1996) Nucleic acids II: the polymerase chain reaction. In: Hillis, D.M., Moritz, C. & Mable, B.K. (Eds.), Molecular Systematics. Sinauer Associates, Sunderland, Massachusetts, pp. 205–247.
- Perseke, M., Hetmank, J., Bernt, M., Stadler, P.F., Schlegel, M. & Bernhard, D. (2011) The enigmatic mitochondrial genome of Rhabdopleura compacta (Pterobranchia) reveals insights into selection of an efficient tRNA system and supports monophyly of Ambulacraria. BMC Evolutionary Biology, 11 (134), 1‒12. https://doi.org/10.1186/1471-2148/11/134
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
- Ramírez-Guerrero, G.M., Kocot, K.M. & Cameron, C.B. (2020) Zooid morphology and molecular phylogeny of the graptolite Rhabdopleura annulata (Hemichordata, Pterobranchia) from Heron Island, Australia. Canadian Journal of Zoology, 98, 844–849. https://doi.org/10.1139/cjz-2020-0049
- Rickards, R.B., Chapman, A.J. & Temple, J.T. (1984) Rhabdopleura hollandi, a new pterobranch hemichordate from the Silurian of the Llandovery district, Powys, Wales. Proceedings of the Geological Association, 95, 23‒28. https://doi.org/10.1016/S0016-7878(84)80017-6
- Rigby, S. (1994) Erect tube growth in Rhabdopleura compacta (Hemichordata: Pterobranchia) from off Start Point, Devon. Journal of Zoology, London, 233, 449‒455. https://doi.org/10.1111/j.1469-7998.1994.tb05276.x
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Zoology, 61, 539‒542. https://doi.org/10.1093/sysbio/sys029
- Rubinoff, D. & Holland, B.S. (2005) Between two extremes: Mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference. Systematic Zoology, 54, 952‒961. https://doi.org/10.1080/10635150500234674
- Sars, G.O. (1872) On some remarkable forms of animal life from the great deeps off the Norwegian coast I. Partly from posthumous manuscripts of the late Professor Dr. Michael Sars. University-Program for the 1st half-year 1869. Brøgger & Christie, Cristiania, 82 pp., 6 pls. https://doi.org/10.5962/bhl.title.8861
- Sars, G.O. (1874) On Rhabdopleura mirabilis (M. Sars). Quarterly Journal of Microscopical Science, New Series, 14, 23‒44, pl. 1. https://doi.org/10.1242/jcs.s2-14.53.23
- Sato, A., Bishop, J.D.D. & Holland, P.W.H. (2008) Developmental biology of pterobranch hemichordates: history and perspectives. Genesis, 46, 587‒591. https://doi.org/10.1002/dvg.20395
- Schepotieff, A. (1907) Die Pterobranchier. Anatomische und histologische Untersuchungen über Rhabdopleura normanii Allman und Cephalodiscus dodecalophus M’Int. 1. Teil. Rhabdopleura normanii Allman. Die Anatomie von Rhabdopleura. Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere, 23, 463–534, pls. 25–33. https://doi.org/10.5962/bhl.part.21044
- Schepotieff, A. (1909) Die Pterobranchier des Indischen Ozeans. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere, 28, 429–448, pls. 7–8.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D.J., Hartenstein, V., Eliceira, K., Tomcak, P. & Cardona, A. (2012) Fiji: an open-source platform for biological-image analysis. Nature Methods, 9, 676–682. https://doi.org/10.1038/nmeth.2019
- Sennikov, N.V. (2016) Morphology of the exoskeleton and soft tissues of Cambrian rhabdopleurids. Paleontological Journal, 50, 1626–1636. https://doi.org/10.1134/S0031030116140070
- Simakov, O., Kawashima, T., Marlétaz, F., Jenkins, J., Koyanagi, R., Mitros, T., Hisata, K., Bredeson, J., Shoguchi, E., Gyoja, F., Yue, J.-X., Chen, Y.-C., Freeman, R.M., Sasaki, A., Hikosaka-Katayama, T., Sato, A., Fujie, M., Baughman, K.W., Levine, J., Gonzalez, P., Cameron, C., Fritzenwanker, J.H., Pani, A.M., Goto, H., Kanda, M., Arakaki, N., Yamasaki, S., Qu, J., Cree, A., Ding, Y., Dinh, H.H., Dugan, S., Holder, M., Jhangiani, S.N., Kovar, K.L., Lee, S.L., Lewis, L.R., Morton, D., Nazareth, L.V., Okwuonu, G., Santibanez, J., Chen, R., Richards, S., Muzny, D.M., Gillis, A., Peshkin, L., Wu, M., Humphreys, T., Su, Y.-H., Putnam, N.H., Schmutz, J., Fujiyama, A., Yu, J.-K., Tagawa, K., Worley, K.C., Gibbs, R.A., Kirschner, M.W., Lowe, C.J., Satoh, N., Rokhsar, D.S. & Gerhart, J. (2015) Hemichordate genomes and deuterostome origins. Nature, 257, 459–465. k https://doi.org/10.1038/nature16150
- Shepherd, S.A. (1977) Acorn worms and a pterobranch (phylum Hemichordata). In: Shepherd, S.A. & Davies, M. (eds), Marine Invertebrates of Southern Australia Part III. Flora and Fauna of South Australia Handbooks Committee for South Australian Research and Development Institute, Adelaide, pp. 1028‒1039.
- Stebbing, A.R.D. (1970a) The status and ecology of Rhabdopleura compacta (Hemichordata) from Plymouth. Journal of the Marine Biological Association of the United Kingdom, 50, 209‒221. https://doi.org/10.1017/S0025315400000722
- Stebbing, A.R.D. (1970b) Aspects of the reproduction and life cycle of Rhabdopleura compacta (Hemichordata). Marine Biology, 5, 205‒212. https://doi.org/10.1007/bf00346908
- Strano, F., Micaroni, V., Beli, E., Mercurio, S., Scarì, G., Pennati, R. & Piraino, S. (2019) On the larva and the zooid of the pterobranch Rhabdopleura recondita Beli, Cameron and Piraino, 2018 (Hemichordata, Graptolithina). Marine Biodiversity, 49, 1657‒1666. https://doi.org/10.1007/s12526-018-0933-2
- Swalla, B.J. & van der Land, J. (2023) Hemichordata World Database. Rhabdopleura Allman, 1869. Accessed through: World Register of Marine Species. Available from: https://www.marinespecies.org/aphia.php?p=taxdetails&id=137594 (accessed 15 August 2023)
- Suyama, M., Torrents, D. & Bork, P. (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Research, 34, W609–W612. https://doi.org/10.1093/nar/gkl315
- Tassia, M.G., Cannon, J.T., Konikoff, C.E., Shenkar, N., Halanych, K.M. & Swalla, B.J. (2016) The global diversity of Hemichordata. PLoS ONE, 11 (10), e0162564, 1‒16. https://doi.org/10.1371/journal.pone.0162564
- Urbanek, A. & Dilly, P.N. (2000) The stolon system in Rhabdopleura compacta (Hemichordata) and its phylogenetic implications. Acta Palaeontologica Polonica, 45, 201–226.
- Watling, L., Guinotte, J., Clark, M.R. & Smith, C.R. (2013) A proposed biogeography of the deep ocean floor. Progress in Oceanography, 111, 91‒112. https://doi.org/10.1016/j.pocean.2012.11.003