Abstract
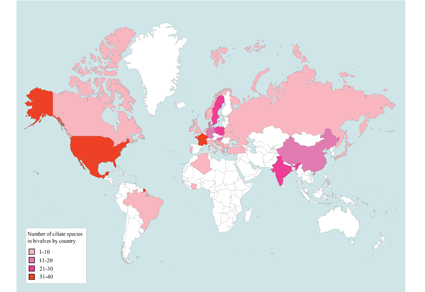
The shells, mantle cavities and various organs of mollusks serve as suitable habitats for symbiotic ciliates, as commensal, epibiotic and parasitic. With about 80,000 species, molluscans are distributed in freshwater, marine and terrestrial habitats; symbiotic ciliates have been recorded in bivalves, gastropods and polyplacophorans; however these records have not been integrated in comprehensive revisions. The goal of this work is to provide an updated checklist of the ciliates involved as symbionts of bivalve molluscs worldwide. Available records of symbiotic species of ciliates were compiled and checked, along with their molluscan hosts and localities. We obtained data for 165 species of ciliates as symbionts of 158 bivalve hosts, distributed in 31 countries, regions and seas. This is the first comprehensive study to review the biodiversity of ciliates associated with bivalves and show that only a small fraction of this class of molluscs has been studied in terms of their symbiotic relationships with ciliates.
References
- Antipa, G.A. & Dolan, J. (1985) Mytilophilus pacificae n. g., n. sp.: a new mytilid endocommensal ciliate (Scuticociliatida). Transactions of the American Microscopical Society, 104, 360–368. https://doi.org/10.2307/3226489
- Antipa, G.A. & Small, E.B. (1971) The occurrence of thigmotrichous ciliated protozoa inhabiting the mantle cavity of unionid molluscs of Illinois. Transactions of the American Microscopical Society, 90, 463–472. https://doi.org/10.2307/3225461
- Antipa, G.A. & Hatzidimitriou, G. (1981) Morphogenesis in Conchophthirus curtus: a study of the morphological events associated with binary fission. Journal of Protozoology, 28, 206–214. https://doi.org/10.1111/j.1550-7408.1981.tb02834.x
- Antipa, G.A., Strüder-Kypke, M.C. & Lynn, D.H. (2020) Molecular phylogeny, taxonomic relationships and Nort American distribution of Conchophthirus (Conchophthiridae, Scuticociliatia). Aquatic Ecosystem Health & Management, 23, 58–68. https://doi.org/10.1080/14634988.2020.1735919
- Aristeo-Hernández, J. (2017) Estudio de los ciliados asociados a moluscos dulceacuícolas (Bivalvia y Gastropoda) de cuerpos de agua continentales de Chiapas y Oaxaca, México. Master´s thesis, Universidad Nacional Autónoma de México, Mexico City, 111 pp. [https://repositorio.unam.mx/contenidos/102484]
- Barillé, L., Le Bris, A., Méléder, V., Launeau, P., Robin, M., Louvrou, I. & Ribeiro, L. (2017) Photosynthetic epibionts and endobionts of Pacific oyster shells from oyster reefs in rocky versus mudflat shores. Plos One, 12 (9), e0185187. https://doi.org/10.1371/journal.pone.0185187
- Berger, J. & Hatzidimitriou, G. (1978) Multivariate morphometric analyses of demic variation in Ancistrum mytili (Ciliophora: Scuticociliatida) comensal in two mytilid pelecypods. Protistologica, 14, 133–153.
- Biswas, T. & Bandyopadhyay, P.K. (2016) First record of protozoan parasites, Tetrahymena rostrata and Callimastix equi from the edible oyster in Sundarbans region of West Bengal, India. Journal of Parasitic Diseases, 40, 971–975. https://doi.org/10.1007/s12639-014-0617-1
- Boitsova, I.L. (1976) Peritricha on the bottom of aquatic invertebrates. Vestnik Leningradskogo Universiteta. Biologiia, 31, 39–49.
- Borisanova, A.O., Chernyshev, A.V. & Ekimova, I.A. (2018) Deep-sea Entoprocta from the Sea of Okhotsk and the adjacent open Pacific abyssal area: New species and new taxa of host animals. Deep Sea Research Part II: Topical Studies in Oceanography, 154, 87–98. https://doi.org/10.1016/j.dsr2.2017.11.010
- Boshko, E. (1987) Epistylis borysthenicus sp. n. (Peritrichia, Epistylididae) infusoria from molluscs of the genera Unio and Anodonta in reservoirs of the Dnieper River basin. Zoologicheskii Zhurnal, 66 (2), 295–298.
- Boshko, E. (1993) New species of ciliophoran genus Mantoscyphidia (Peritrichia) from freshwater molluscs. Vestnik Zoologii, 6, 14–16. [https://archive.org/details/vestnzool1993n69393603boshko]
- Botes, H. (1999) Sessiline ciliophorans associated with Haliotis species (Mollusca: Archaeogastropoda) from the South Coast of South Africa. Magister thesis, University of the Orange Free State, Bloemfontein, 230 pp. [http://hdl.handle.net/11660/5938]
- Bower, S.M. & Meyer, G.R. (1993) Stegotricha enterikos gen nov., sp. nov. (class Phyllopharyngea, order Rhynchodida), a parasitic ciliate in the digestive gland of Pacific oysters (Crassostrea gigas), and its distribution in British Columbia. Canadian Journal of Zoology, 71, 2005–2017. https://doi.org/10.1139/z93-285
- Brusca, R.C., Moore, W. & Shuster, S.M. (2016) Invertebrates. Sinauer Associates, Sunderland, Massachusetts, 1104 pp. https://doi.org/10.1080/10635150490472968
- Burlakova, L.E., Karatayev, A.Y. & Molloy, D.P. (1998) Field and laboratory studies of zebra mussel (Dreissena polymorpha) infection by the ciliate Conchophthirus acuminatus in the Republic of Belarus. Journal of Invertebrate Pathology, 71, 251–257. https://doi.org/10.1006/jipa.1997.4728
- Burlakova, L.E., Padilla, D.K., Karatayev, A.Y. & Minchin, D. (2006) Endosymbionts of Dreissena polymorpha in Ireland: evidence for the introduction of adult mussels. Journal of Molluscan Studies, 72, 207–210. https://doi.org/10.1093/mollus/eyi067
- Carss, D.N., Brito, A.C., Chainho, P., Ciutat, A., Montaudouin, X., Otero, R.M.F., Filgueira, M.I., Garbutt, A., Goedknegt, M.A., Lynch, S.A., Mahony, K.E., Maire, O., Malham. S.K., Orvain, F., Olivier, A. van der S. & Jones, L. (2020) Ecosystem services provided by non-cultured shellfish species: the common cockle Cerastoderme edule. Marine Environmental Research, 158, 104931. https://doi.org/10.1016/j.marenvres.2020.104931
- Cépéde, C. & Poyarkoff, E. (1909) Sur in infusoire astome Cepedella hepatica Poyarkoff parasite du foie des Cyclas (S. corneum L.). Bulletin Scientifique de la France et de la Belgique, 43, 463–475. [https://www.biodiversitylibrary.org/item/40689#page/507/mode/1up]
- Chatterjee, T. & Dovgal, I.V. (2020) A checklist of ciliate epibionts (Ciliophora) found on bryozoans. Zootaxa, 4896 (4), 547–559. https://doi.org/10.11646/zootaxa.4896.4.6
- Chatterjee, T., Dovgal, I. & Fernandez-Leborans, G. (2019) A checklist of suctorian epibiont ciliates (Ciliophora) found on meiobenthic marine nematodes. Journal of Natural History, 53 (33–34), 2133–2143. https://doi.org/10.1080/00222933.2019.1692085
- Chuseve, R., Mastitsky, S.E. & Zaiko, A. (2012) First report of endosymbionts in Dreissena polymorpha from the brackish Curonian Lagoon, SE Baltic Sea. Oceanologia, 54, 701–713. https://doi.org/10.5697/oc.54-4.701
- Çinar, M.E., Bakir, K., Öztürk, B., Doğan, A., Açik, Ş., Kirkim, F., Dağli, E., Kurt, G., Evcen, A., Koçak, F. & Bitlis, B. (2020) Spatial distribution pattern of macroinvertebrates associated with the black mussel Mytilus galloprovincialis (Mollusca: Bivalvia) in the Sea of Marmara. Journal of Marine Systems, 211, 103402. https://doi.org/10.1016/j.jmarsys.2020.103402.
- Clamp, J.C. & Lynn, D.H. (2017) Investigating the biodiversity of ciliates in the “Age of Integration”. European Journal of Protistology, 61, 314–322. https://doi.org/10.1016/j.ejop.2017.01.004
- Conn, D.B., Simpson, S.E., Minchin, D. & Lucy, F.E. (2008) Occurrence of Conchophthirus acuminatus (Protista: Ciliophora) in Dreissena polymorpha (Mollusca: Bivalvia) along the River Shannon, Ireland. Biological Invasions, 10, 149–156. https://doi.org/10.1007/s10530-007-9118-9
- Costa, P.M., Carreira, S., Lobo, J. & Costa, M.H. (2012) Molecular detection of prokaryote and protozoan parasites in the commercial bivalve Ruditapes decussatus from southern Portugal. Aquaculture, 370–371, 61–67. https://doi.org/10.1016/j.aquaculture.2012.10.006
- Costa, F.S., Dias, R.J.P. & Rossi, M.F. (2021) Macroevolutionary analyses of ciliates associated with hosts support high diversification rates. International Journal for Parasitology, 51 (11), 967–976. https://doi.org/10.1016/j.ijpara.2021.03.006
- Da Silva-Neto, I. (1992) Observations sur la structure et l´ultrastructure du cilié Myxophthirus anomalocardiae gen. nov., sp. nov. (Scuticociliatida, Thigmophryidae), parasite du bivalve Anomalocardia brasiliana Gmelin, 1791. European Journal of Protistology, 28, 421–429. https://doi.org/10.1016/S0932-4739(11)80006-4
- De Morgan, W. (1925) Some marine ciliates living in the laboratory tanks at Plymouth, with a description of a new species, Holophrya coronata. Journal of the Marine Biological Association of the United Kingdom, 13, 600–658. https://doi.org/10.1017/S0025315400008080
- De Puytorac, P., Grain, J., Groliére, C.A. & López-Ochoterena, E. (1978) Sur l´ultrastructure du cilié Proboveria rangiae sp. nov. endocommensal du lamellibranche Rangia cuneata. Protistologica, 14, 503–512.
- Deshmukh, N.Z., More, B.V., Jaid, E.L. & Nikam, S.V. (2011) Study of ciliate commensals from the gills of freshwater bivalves (Molluscs: Bivalvia) of Jayakwadi Dam, Paithan, (M.S.), India. Journal of Ecobiotechnology, 3, 9–12. [https://updatepublishing.com/journal/index.php/jebt/article/view/123]
- Dolan, J. & Antipa G.A. (1985) Comparative stomatogenesis of two encommensal scuticociliates, Peniculistoma mytili and Mytilophilus pacificae from marine mytilid mussels. Protistologica, 21, 323–332.
- Donadi, S., Heide, T. van der, Zee, E.M. van der, Eklöf, J.S., Kopel, T.P., Weerman, E.J., Piersma, T., Olff, H. & Eriksson, B.K. (2013) Cross-habitat interactions among bivalve species control community structure on intertidal flats. Ecology, 94, 489‒498. https://doi.org/10.1890/12-0048.1
- Dos Santos, A.M.T. & Coimbra, J. (1995) Growth and production of raft-cultured Mytilus edulis L., in Ria de Aveiro: gonad symbiotic infestation. Aquaculture, 132, 195–211. https://doi.org/10.1016/0044-8486(94)00352-O
- Dovgal, I., Chatterjee, T. & Ingole, B. (2008) An overview of suctorian ciliates (Ciliophora, Suctorea) as epibionts of halacarid mites (Acari, Halacaridae). Zootaxa, 1810 (1), 60–68. https://doi.org/10.11646/zootaxa.1810.1.4
- Fenchel, T. (1964a) On Ancistrum caudatum sp. nov. and Hypocomides modiolariae Chatton & Lwoff (Ciliata, Thigmotrichida) from the lamellibranch Musculus niger (Gray). Ophelia, 1, 113–120. https://doi.org/10.1080/00785326.1964.10416274
- Fenchel, T. (1964b) On the morphology, morphogenesis and systematics of Thigmophrya Ch. Lw. (Ciliata, Thigmotrichida) with a description of T. saxicavae sp. n. Acta Protozoologica, 2, 113–121.
- Fenchel, T. (1964c) Gullmarella faurei n. g., n. sp., a holotrichous ciliate from the intestine of lamellibranchs. Cahiers de Biologie Marine, 5, 311–318.
- Fenchel, T. (1965) Ciliates from Scandinavian molluscs. Ophelia, 2, 71–174.
- Fernandez-Leborans, G. & Tato-Porto, M.L. (2000a) A review of the species of protozoan epibionts of crustaceans. I. Peritrich ciliates. Crustaceana, 73, 643–683. https://doi.org/10.1163/156854000504705
- Fernandez-Leborans, G. & Tato-Porto, M.L. (2000b) A review of the species of protozoan epibionts of crustaceans. II. Suctorian ciliates. Crustaceana, 73, 1205–1237. https://doi.org/10.1163/156854000505209
- Fokin, S. & Karpov, S. (1995) Bacterial endocytobionts inhabiting the pereinuclear space of Protista. Endocytobiosis & Cell Research, 11, 81–94. [https://api.semanticscholar.org/CorpusID:88159963]
- Fokin, S.I., Giamberini, L., Molloy, D.P. & de Vaate, A. (2003) Bacterial endocytobionts within endosymbiotic ciliates in Dreissena polymorpha (Lamellibranchia: Mollusca). Acta Protozoologica, 42, 31–39. [https://api.semanticscholar.org/CorpusID:39025004]
- Gao, F., Fan, X., Yi, Z., Strüder-Kypke, M. & Song, W. (2010) Phylogenetic consideration of two scuticociliate genera, Philasterides and Boveria (Protozoa, Ciliophora) based on 18 S rRNA gene sequences. Parasitology International, 59, 549–555. https://doi.org/10.1016/j.parint.2010.07.002
- Gao, F., Strüder-Kypke, M., Yi, Z., Miao, M., Al-Farraj, S.A. & Song, W. (2012) Phylogenetic analysis and taxonomic distinction of six genera of pathogenic scuticociliates (Protozoa, Ciliophora) inferred from small-subunit rRNA gene sequences. International Journal of Systematic and Evolutionary Microbiology, 62, 246–256. https://doi.org/10.1099/ijs.0.028464-0
- Gauthier, J.D., Soniat, T.M. & Rogers, J.S. (1990) A parasitological survey of oysters along salinity gradients in coastal Louisiana. Journal of the World Aquaculture Society, 21, 105–115. https://doi.org/10.1111/j.1749-7345.1990.tb00530.x
- Hatzidimitriou, G. & Berger, J. (1977) Morphology and morphogenesis of Ancistrum mytili (Scuticociliatida: Thigmotrichina), a commensal ciliate of mytilid pelecypods. Protistologica, 13, 477–495. [https://api.semanticscholar.org/CorpusID:89339111]
- Haszprunar, G. (2020) Mollusca (Molluscs). eLS, 1, 565‒571. https://doi.org/10.1038/npg.els.0001598
- Hiebert, T.C. (2015) Adula californiensis. In: Hiebert, T.C., Butler, B.A. & Shanks, A.L, (Eds.), Oregon Estuarine Invertebrates: Rudys´Illustrated Guide to commons species. 3rd Edition. University of Oregon Libraries and Oregon Institute of Marine Biology, Charleston, Oregon, 6 pp. [http://hdl.handle.net/1794/12740]
- Hiebert, T.C. (2016) Mytilus trossulus. In: Hiebert, T.C., Butler, B.A. & Shanks, A.L, (Eds.), Oregon Estuarine Invertebrates: Rudys´Illustrated Guide to commons species. 3rd Edition. University of Oregon Libraries and Oregon Institute of Marine Biology, Charleston, Oregon, 12 pp. [http://hdl.handle.net/1794/12912]
- Hillman, R.E. (1979) Encystment of the ciliate Boveria teredini in the tissues of the molluscan woodborer Bankia gouldi in Barnegat Bay, New Jersey. Journal of Invertebrate Pathology, 34, 78–83. https://doi.org/10.1016/0022-2011(79)90056-9
- Hirshfield, H.I. (1950) The protozoan fauna of some species of intertidal invertebrates in Southern California. The Journal of Parasitology, 36, 107–112. https://doi.org/10.2307/3273585
- Hu, X. & Song, W. (2002) Studies on the ectocommensal ciliate, Trachelostyla tani nov. spec. (Protozoa: Ciliophora: Hypotrichida) from the mantle cavity of the scallop Chlamys farrei. Hydrobiologia, 481, 173–179. https://doi.org/10.1023/A:1021202230090
- Huang, S., Edie, S.M., Collins, K.S., Crouch, M.A., Roy, K. & Jablonski, D. (2023) Diversity, distribution and intrinsic extinction vulnerability of exploited marine bivalves. Nature Communications, 14, 4639. https://doi.org/10.1038/s41467-023-40053-y
- Ikeda, I. & Ozaki, Y. (1918) Notes on a new Boveria species, Boveria labialis n. sp. Journal of the College of Science, Tokyo Imperial University, 40, 1–25. [https://www.biodiversitylibrary.org/page/7092641#page/411/mode/1up]
- Issel, R. (1903) Ancistridi del Golfo di Napoli. Studio monografico sopra una nuova famiglia di cigliati commensali di molluschi marini. Mittheilungen aus der Zoologischen Station zu Neapal, 16, 63–108. [https://www.biodiversitylibrary.org/page/9665048#page/75/mode/1up]
- Jamadar, Y.A. & Choudhury, A. (1988) Ciliates of some marine and estuarine molluscs from Indian coastal region. Technical Monograph, Zoological Survey of India, 12, 1–79. [https://archive.org/details/dli.zoological.tcm.012]
- Karatayev, A.Y., Molloy, D.P. & Burlakova, L.E. (2000a) Seasonal dynamics of Conchophthirus acuminatus (Ciliophora, Conchophthiridae) infection in Dreissena polymorpha and D. bugensis (Bivalvia, Dreissenidae). European Journal of Protistology, 36, 397–404.
- Karatayev, A.Y., Burlakova, L.E., Molloy, D.P. & Volkova, L.K. (2000b) Endosymbionts of Dreissena polymorpha (Pallas) in Belarus. International Review of Hydrobiology, 85, 543–559. https://doi.org/10.1002/1522-2632(200011)85:5/6<543::AID-IROH543>3.0.CO;2-3
- Karatayev, A.Y., Mastitsky, S.E., Molloy, D.P. & Burlakova, L.E. (2003a) Patterns of emergence and survival of Conchophthirus acuminatus (Ciliophora: Conchophthiridae) from Dreissena polymorpha (Bivalvia: Dreissenidae). Journal of Shellfish Research, 22, 495–500.
- Karatayev, A.P., Mastitsky, S.E., Burlakova, L.E., Molloy, D.P. & Vezhnovets, G.G. (2003b) Seasonal dynamics of endosymbiotic ciliates and nematodes in Dreissena polymorpha. Journal of Invertebrate Pathology, 83, 73–82. https://doi.org/10.1016/S0022-2011(03)00043-0
- Karatayev, A.P., Burlakova, L.E., Molloy, D.P. & Mastitsky, S.E. (2007) Dreissena polymorpha and Conchophthirus acuminatus: what can we learn from host-commensal relationships. Journal of Shellfish Research, 26, 1153–1160. https://doi.org/10.2983/0730-8000(2007)26[1153:DPACAW]2.0.CO;2
- Khan, M.A. (1969) Fine structure of Ancistrocoma pelseneeri (Chatton et Lwoff), a rhynchodine thigmotrichid ciliate. Acta Protozoologica, 7, 29–47.
- Khan, M.A. (1970) Electron microscopic studies of Thigmophrya macomae Ch. et Lw., an arhynchodine thigmotrichid ciliate. Acta Protozoologica, 8, 67–77.
- Kidder, G.W. (1933) On the genus Ancistruma Strand (Ancistrum Maupas). I. The structure and division of A. mytili Quenn, and A. isseli Kahl. The Biological Bulletin, The Marine Biological Laboratory Woods Hole, 64, 1–20. [https://www.biodiversitylibrary.org/partpdf/21299]
- Knight, R. & Thorne, J. (1982) Syncilancistrumina elegantissima (Scuticociliatida: Thigmotrichina), a new genus and species of ciliated protozoon from Pholas dactylus (Mollusca: Bivalvia), the common piddock. Protistologica, 18, 53–66.
- Kozloff, E.N. (1946a) Studies on ciliates of the family Ancistrocomidae Chatton and Lwoff (order Holotricha, suborder Thigmotricha). I. Hypocomina tegularum sp. nov. and Crebricoma gen. nov. The Biological Bulletin, The Marine Biological Laboratory Woods Hole, 90, 1–7. https://doi.org/10.2307/1538118
- Kozloff, E.N. (1946b) Studies on ciliates of the family Ancistrocomidae Chatton and Lwoff (order Holotricha, suborder Thigmotricha). II. Hypocomides mytili Chatton and Lwoff, Hypocomides botulae sp. nov., Hypocomides parva sp. nov., Hypocomides kelliae sp. nov., and Insignocoma venusta gen. nov., sp. nov. The Biological Bulletin, The Marine Biological Laboratory Woods Hole, 90, 200–212. https://doi.org/10.2307/1538118
- Kozloff, E.N. (1957) A species of Tetrahymena parasitic in the renal organ of the slug Deroceras reticulatum. Journal of Protozoology, 4, 75–79. https://doi.org/10.1111/j.1550-7408.1957.tb02490.x
- Kozloff, E.N. & Norenberg, J.P. (2013) Raabella concinna sp. nov. and Hypocomina obstipa sp. nov., ciliates of the family Ancistrocomidae parasitizing marine molluscs on the Pacific coast of North America. Cahiers de Biologie Marine, 54, 175–180. [http://www.sb-roscoff.fr/CBM/]
- Krapivin, V.A., Bagrov, S.V. & Varfolomeeva, M.A. (2018) Effect of tidal level on abundance of symbionts in the White Sea blue mussel. Diseases of Aquatic Organisms, 130, 131–144. https://doi.org/10.3354/dao03259
- Kuidong, X., Yanli, L. & Song, W. (1995) Morphological studies on Trichodina jadranica Raabe, 1958, a scallop ciliate parasite (Protozoa: Ciliophora: Peritricha). Journal of Ocean University of Qingdao, 25, 321–326. [https://api.semanticscholar.org/CorpusID:202859497]
- Lacoste, É., Raimbault, P., Harmelin-Vivien, M. & Gaertner-Mazouni, N. (2016) Trophic relationships between the farmed pearl oyster Pinctada margaritifera and its epibionts revealed by stable isotopes and feeding experiments. Aquaculture Environment Interactions, 8, 55–66. https://doi.org/10.3354/aei00157
- Laird, M. (1961) Microecological factors in oyster epizootics. Canadian Journal of Zoology, 39, 449–485. https://doi.org/10.1139/z61-050
- Landacre, F.L. (1908) The protozoa of Sandusky Bay and vicinity. Proceedings of the Ohio State Academy of Science, 4, 423–472. [https://www.biodiversitylibrary.org/item/50902#page/465/mode/1up]
- Latruelle. F., Molloy, D.P. & Ovcharenko, M.A. (1999) Histological analysis of mantle-cavity ciliates in Dreissena polymorpha: their location, symbiotic relationship, and distinguishing morphological characteristics. Journal of Shellfish Research, 18, 251–257. https://doi.org/10.1645/0022-3395(2002)088[0856:HAOTID]2.0.CO;2
- Lom, J. & Kozloff, E.N. (1968) Observations on the ultrastructure of the suctorial tube of ancistrocomid ciliates. Folia Parasitologica, Praha, 15, 291–308.
- Lom, J., Corliss, J.O. & Noirot-Timothée, C. (1968) Observations on the ultrastructure of the buccal apparatus in thigmotrich ciliates and their bearing on thigmotrich-peritrich affinities. Journal of Protozoology, 15, 824–840. https://doi.org/10.1111/j.1550-7408.1968.tb02222.x
- Lopes-Lima, M., Burlakova, L.E., Karatayev, A.Y., Mehler, K., Seddon, M. & Sousa, R. (2018) Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia, 810, 1–14. https://doi.org/10.1007/s10750-017-3486-7
- Lopes, E.P., Monteiro, N. & Santos, A.M. (2020) Epibiotic assemblages on the pen shell Pinna rudis (Bivalvia, Pinnidae) at Matiota Beach, São Vicente Island, Cabo Verde, African Journal of Marine Science, 42, 13‒21. https://doi.org/10.2989/1814232X.2019.1700826
- López-Ochoterena, E. (1999) Diversidad protozoológica de México. II. Especies asociadas a moluscos. Revista de la Sociedad Mexicana de Historia Natural, 49, 105–108.
- Lynn, D.H. (2008) The Ciliated Protozoa. Characterization, classification, and guide to the literature. Springer, New York, New York, 605 pp.
- Mackinnon, D.L. & Ray H.N. (1931) Notes on the ciliate Boveria stevensi Issel from Galeomma turtoni Sowerby at Plymouth. Journal of the Marine Biological Association of the United Kingdom, 17, 577–582. https://doi.org/10.1017/S0025315400051043
- Madrazo-Garibay, M. & López-Ochoterena, E. (1985) Protozoarios ciliados de México. XXVII. Aspectos biológicos de siete especies asociadas a Crassostrea rizophorae (Guilding) (Mollusca: Bivalvia), recolectadas en la laguna de Términos, Campeche. Anales del Instituto de Ciencias del Mar y Limnología Universidad Nacional Autónoma de México, 12, 213–220. [https://biblat.unam.mx/pt/revista/anales-del-instituto-de-ciencias-del-mar-y-limnologia-unam/articulo/protozoarios-ciliados-de-mexico-xxvii-aspectos-biologicos-de-siete-especies-asociadas-a-crassostrea-rizophorae-guilding-mollusca-bivalvia-recolectadas-en-la-laguna-de-terminos-campeche]
- Madrazo-Garibay, M. & López-Ochoterena, E. (1986) Protozoarios ciliados de México. XXIX. Aspectos biológicos de seis especies asociadas a Crassostrea virginica (Gmelin) (Mollusca, Bivalvia) de la laguna de Términos, Campeche, México. Anales del Instituto de Ciencias del Mar y Limnología Universidad Nacional Autónoma de México, 13, 39–44.
- Madrazo-Garibay, M. & López-Ochoterena, E. (1988a) Protozoarios ciliados de México. XXX. Descripción y sistemática de algunas especies asociadas a tres almejas comerciales (Mollusca, Bivalvia) del sistema fluvio-lagunar Atasta-Pom, Campeche, México. Anales del Instituto de Ciencias del Mar y Limnología Universidad Nacional Autónoma de México, 15, 55–64.
- Madrazo-Garibay, M. & López-Ochoterena, E. (1988b) Protozoarios ciliados de México. XXXI. Siete especies del género Scyphidia Dujardin (Peritrichida, Oligohymenophorea) y su asociación con almejas comestibles (Mollusca, Bivalvia) de la laguna Pom Campeche. Anales del Instituto de Ciencias del Mar y Limnología Universidad Nacional Autónoma de México, 15, 223–228.
- Madrazo-Garibay, M., López-Ochoterena, E., Rico-Ferrat, G. & Serrano-Limón, G. (1990) Especies del phylum Ciliophora asociadas a animales silvestres, estudiadas en México. III. Relación taxonómica y bibliográfica. Anales del Instituto de Biología Universidad Nacional Autónoma de México, Serie Zoología, 61, 449–456. [https://biblat.unam.mx/en/revista/anales-del-instituto-de-biologia-unam-serie-zoologia/articulo/especies-del-phylum-ciliophora-asociadas-a-animales-domesticos-estudiadas-en-mexico-vi-relacion-taxonomica-y-bibliografica]
- Mastitsky, S.E. (2012) Infection of Dreissena polymorpha (Bivalvia: Dreissenidae) with Conchophthirus acuminatus (Ciliophora: Conchophthiridae) in lakes of different trophy. BioInvasions Records, 1, 161–169. https://doi.org/10.3391/bir.2012.1.3.02
- Mastitsky, S.E., Lucy, F. & Gagarin, V.G. (2008) First report of endosymbionts in Dreissena polymorpha from Sweden. Aquatic Invasions, 3, 83–86. https://doi.org/10.3391/ai.2008.3.1.13
- Mieczan, T. & Rudyk-Leuska, N. (2019) Seasonal dynamics of the epibiont food web on Unio tumidus (Philipsson, 1788) in a eutrophic reservoir. European Journal of Protistology, 69, 138–150. https://doi.org/10.1016/j.ejop.2019.04.003
- Minchin, D. & Holmes, J.M.C. (2008) The Ponto-Caspian mysid, Hemimysis anomala G. O. Sars, 1907 (Crustacea), arrives in Ireland. Aquatic Invasions, 3, 257–259. https://doi.org/10.3391/ai.2008.3.2.19
- Minguez, L., Meyer, A., Molloy, D.P. & Giambérini, L. (2009) Interactions between parasitism and biological responses in zebra mussels (Dreissena polymorpha): importance in ecotoxicological studies. Environmental Research, 109, 843–850. https://doi.org/10.1016/j.envres.2009.07.012
- Minguez, L., Molloy, D.P., Guérold, F. & Giambérini, L. (2011) Zebra mussel (Dreissena polymorpha) parasites: potentially useful bioindicators of freshwater quality? Water Research, 45, 665–673. https://doi.org/10.1016/j.watres.2010.08.028
- Minguez, L. & Giambérini, L. (2012) Seasonal dynamics of zebra mussel parasite populations. Aquatic Biology, 15, 145–151. https://doi.org/10.3354/ab00418
- Molloy, D.P., Lynn, D.H. & Giambérini, L. (2005) Ophryoglena hemophaga n. sp. (Ciliophora: Ophryoglenidae): a parasite of the digestive gland of zebra mussels Dreissena polymorpha. Diseases of Aquatic Organisms, 65, 237–243. https://doi.org/10.3354/dao065237
- Molloy, D.P., Giambérini, L., Burlakova, L.E., Karatayev, A.Y., Cryan, J.R., Trajanovski, S.L. & Trajanovska, S.P. (2010) Investigation of the endosymbionts of Dreissena stankovici with morphological and molecular confirmation of host species. In: Van der Velde, G., Rajagopal, S. & bij de Vaate, A. (Eds.), Zebra mussels in Europe. Backhuys Publishers, Leiden, pp. 227–478.
- Morado, J.M. & Small, E.B. (1995) Ciliate parasites and related diseases of Crustacea: a review. Reviews in Fisheries Science, 3 (4), 275–354. https://doi.org/10.1080/10641269509388575
- Nandi, N.C., Das, A.K. & Sarkar, N.C. (1993) Protozoa fauna of Sundarban mangrove ecosystem. Records of the Zoological Survey of India, 93, 83–101. https://doi.org/10.26515/rzsi/v93/i1-2/1993/160864
- Oishi, T. (1978) On a new ciliate, Ancistrum edajimanum n. sp., from a bivalve, Tapes philippinarum (Adams et Reeve). Proceedings of the Japanese Society of Systematic Zoology, 14, 1–4. https://doi.org/10.19004/pjssz.14.0_1
- Ovcharenko, M. (2000) Symbiocoenosis of Dreissena polymorpha and D. bugensis. Folia Malacologica, 8, 285–298.
- Özer, A. & Güneydag, S. (2013) First report of some parasites from the Mediterranean mussel, Mytilus galloprovincialis Lamarck, 1819, collected from the Turkish Black Sea. Rapports et Proces-verbaux des Reunions de la Commision Internationale pour l´exploration Scientifique de la Mer Mediterranée, 40, 764. https://doi.org/10.3906/zoo-1401-2
- Özer, A. & Güneydag, S. (2014) First report of some parasites from Mediterranean mussel, Mytilus galloprovincialis Lamarck, 1819, collected from the Black Sea coast at Sinop. Turkish Journal of Zoology, 38, 486–490.
- Özer, A. & Güneydag, S. (2015) Seasonality and host-parasite interrelationship of Mytilus galloprovincialis parasites in Turkish Black Sea coasts. Journal of the Marine Biological Association of the United Kingdom, 95, 1591–1599. https://doi.org/10.1017/S0025315415000740
- Penn, J.H. (1958) Studies on ciliates from mollusks of Iowa. Proceedings of the Iowa Academy of Science, 65, 517–534. [https://scholarworks.uni.edu/pias/vol65/iss1/75]
- Peters, H., Van As, L.L., Basson, L. & Van As, J.G. (2004) A new species of Ellobiophrya Chatton et Lwoff, 1923 (Ciliophora: Peritrichia) attached to Mantoscyphidia Jankowski, 1980 (Ciliophora: Peritrichia) species. Acta Protozoologica, 43, 163–172.
- Precht, H. (1935) Epizoen der Kieler Bucht. Nova Acta Leopoldina, 3, 405–474.
- Pryanichnikova, E.G., Tyutin, A.V. & Shcherbina, G.K.H. (2011) Comparative analysis of the structure and fauna of endosymbionts of communities of two dreissenid species (Mollusca, Dreissenidae) in the Upper Volga reservoirs. Inland Water Biology, 4, 203–210. https://doi.org/10.1134/S1995082911020179
- Raabe, Z. (1970a) Ordo Thigmotricha (Ciliata-Holotricha). II. Familia Hemispeiridae. Acta Protozoologica, 7, 117–180.
- Raabe, Z. (1970b) Ordo Thigmotricha (Ciliata-Holotricha). III. Familiae Ancistrocomidae et Sphenopryidae. Acta Protozoologica, 7, 385–463.
- Raabe, Z. (1971) Ordo Thigmotricha (Ciliata-Holotricha). IV. Familia Thigmophryidae. Acta Protozoologica, 9, 121–170.
- Raabe, Z. (1972) Ordo Thigmotricha (Ciliata-Holotricha). V. Familiae Hysterocinetidae et Protoanoplophryidae. Acta Protozoologica, 10, 115–184.
- Richards, B.R., Belmore, C.I. & Hillman, R.E. (1979) Woodborer study associated with the Oyster Creek Generating Station. Annual Report 14893. William F. Clapp Laboratories, Inc., Duxbury, Massachusetts, 22 pp.
- Royer, J., Ropert, M., Mathieu, M. & Costil, K. (2006) Presence of spionid worms and other epibionts in Pacific oysters (Crassostrea gigas) cultured in Normandy, France. Aquaculture, 253, 461–474. https://doi.org/10.1016/j.aquaculture.2005.09.018
- Santhakumari, V. (1973) A brief account of the commensals, associates and predators of the marine wood boring animals. Mahasagar, 6, 178–181.
- Santhakumari, V. (1976) The exposure of the host shipworm to external factors and its effects on the associated organisms. Journal of the Marine Biological Association of India, 18, 637–641.
- Santhakumari, V. (1985) The effect of salinity on some endocommensalic ciliates from shipworms. Fishery Technology, 22, 66–69. [http://hdl.handle.net/1834/33865]
- Santhakumari, V. & Nair, N.B. (1970) Nucleocorbula adherens gen. & sp. nov. (Ciliata. Thigmotrichida) from shipworms. Ophelia, 7, 139–144. https://doi.org/10.1080/00785236.1970.10419294
- Santhakumari, V. & Nair, N.B. (1973) Ciliates from marine wood-boring molluscs. Treubia, 28 (2), 41–58. [http://drs.nio.org/drs/handle/2264/5729]
- Santhakumari, V. & Nair, N.B. (1975) Some observations on the division of five species of commensalic ciliates in relation to water propulsion. Hydrobiologia, 47, 367–380. https://doi.org/10.1007/BF00039583
- Santhakumari, V. & Nair, N.B. (1982) Observations on the parasites and associates of wood boring molluscs and crustaceans of the South-West Coast of India. Fishery Technology, 19, 65–73.
- Schödel, H. (2018) A synopsis of the limnetic epizoic Peritrichia (Ciliophora) on invertebrates in Western-, Middle- and Eastern Europe. Denisia, 41, 47–294.
- Small, E.B. & Antipa, G.A. (1967) Use of endocommensal molluscan ciliated protozoa as indicators of water quality and pollution in Illinos Waters. WRC Technical Report 24. University of Illinois, Chicago, Illinois, 27 pp. [https://www.ideals.illinois.edu/items/93339]
- Song, W. (2000) Morphological and taxonomical studies on some marine scuticociliates from China Sea, with description of two new species, Philasterides armatalis sp. n. and Cyclidium varibonneti sp. n. (Protozoa: Ciliophora: Scuticociliatida). Acta Protozoologica, 39, 295–322. [https://api.semanticscholar.org/CorpusID:88039480]
- Stanley, S.M. (2014) Evolutionary radiation of shallow-water Lucinidae (Bivalvia with endosymbionts) as a result of the rise of seagrasses and mangroves. Geology, 42, 803–806. https://doi.org//10.1130/G35942.1
- Stiller, J. (1931) Die peritrichen infusorien von Tihany und Umgebung. Arbeiten des Ungarischen Biologischen Forschungs Institutes, 4, 171–205.
- Stiller, J. (1941a) Einige Gewässer der Umgebung von Szeged und ihre Peritrichenfauna. Archiv für Hydrobiologie, 38, 313–435.
- Stiller, J. (1941b) Epizoische peritrichen aus dem Balaton. Arbeiten des Ungarischen Biologischen Forschungs Institutes, 1, 211–223. https://doi.org/10.1007/BF00023590
- Tuffrau, M. & Laval-Peuto, M. (1978) Les ciliés endocommensaux d´un taret de côte d´Ivoire, Teredo adami, mollusque Teredinidae. II. Infraciliature et polymorphisme de Biggaria caryoselaginelloides Tchang, 1958 (Hymenostome). Protistologica, 14, 217–224.
- Uzmann, J.R. & Stickney, A.P. (1954) Trichodina myicola n. sp., a peritrichous ciliate from the marine bivalve Mya arenaria L. Journal of Protozoology, 1, 149–155. https://doi.org/10.1111/j.1550-7408.1954.tb00808.x
- Villamil, T., Kauffman, E.G. & Leanza, H.A. (1998) Epibiont habitation patterns and their implications for life habits and orientation among trigoniid bivalves. Lethaia, 31, 43–56. https://doi.org/10.1111/j.1502-3931.1998.tb00489.x
- Wiroonpan, P. & Purivirojkul, W. (2019) New record of Trichodina unionis (Ciliophora, Trichodinidae) from freshwater gastropods in Bangkok, Thailand. Parasite, 26, 1–11. https://doi.org/10.1051/parasite/2019047
- Xu, K. & Song, W. (1997) A morphological study on a new species of gill parasitic ciliate, Urceolaria cheni nov. spec. from the clam Scapharca subcrenata. Journal of Fishery Sciences of China, 5 (3), 13–17.
- Xu, K. & Song, W. (1998) A new ectoparasitic pathogenic ciliate, Hypocomides weihaiensis n. sp. (Ciliophora, Rhynchodida). Journal of Fishery Sciences of China, 5, 11–16.
- Xu, K. & Song, W. (2008) Two trichodinid ectoparasites from marine molluscs in the Yellow Sea, off China, with description of Trichodina caecellae n. sp. (Protozoa: Ciliophora: Peritrichia). Systematic Parasitology, 69, 1–11. https://doi.org/10.1007/s11230-007-9094-6
- Xu, K., Meng, F., Cao, J. & Song, W. (1997) Morphological studies on the thigmotrichine ciliate, Ancistrum crassum Fenchel, 1965 on the gills of short-necked clam. Journal of Ocean University of Qingdao, 27, 466–470.
- Xu, K., Lei, Y., Al-Rasheid, K.A.S. & Song, W. (2011) Two new ectoparasitic ciliates, Sphenophrya solinis sp. nov. and Planeticovorticella paradoxa sp. nov. (Protozoa: Ciliophora), from marine molluscs. Journal of the Marine Biological Association of the United Kingdom, 91, 265–274. https://doi.org/10.1017/S0025315410001967
- Xu, K., Song, W. & Warren, A. (2015) Two new and two poorly known species of Ancistrum (Ciliophora, Scuticociliatia, Thigmotrichida) parasitizing marine molluscs from Chinese coastal waters of the Yellow Sea. Acta Protozoologica, 54, 195–208. https://doi.org/10.4467/16890027AP.15.016.3213
- Yurishinets, V.I. (1999) Some aspects of interactions of Unio tumidus and Unio pictorum (Bivalva, Unionidae) populations with their parasites and commensals. Hydrobiological Journal, 35, 119–122. https://doi.org/10.1615/HydrobJ.v35.i4.120
- Yurishinets, V.I., Ivasyuk, Y.S. & Krasutskaya, N.A. (2008) Experimental infestation of the mollusk Dreissena polymorpha (Bivalvia: Dreissenidae) by the ciliate Conchophthirus acuminatus (Ciliophora: Oligohymenophorea). Hydrobiological Journal, 44, 104–112. https://doi.org/10.1615/HydrobJ.v44.i1.90
- Zhan, Z., Xu, K. & Dunthorn, M. (2012) Evaluating molecular support for and against the monophyly of the Peritrichia and phylogenetic relationships within the Mobilida (Ciliophora, Oligohymenophorea). Zoologica Scripta, 42, 213–226. https://doi.org/10.1111/j.1463-6409.2012.00568.x
- Zhan, Z., Li, J. & Xu, K. (2017) Detection and quantification of two parasitic ciliates Boveria labialis and Boveria subcylindrica (Ciliophora: Scuticociliatia) by fluorescence in situ hybridization. Journal of Eukaryotic Microbiology, 65, 440–447. https://doi.org/10.1111/jeu.12488
- Zhao, Y. & Tang, F. (2007) Trichodinid ectoparasites (Ciliophora: Peritrichia) from Misgurnis anguillicaudatus (Cantor) and Anodonta woodiana (Lea) in China, with descriptions of two new species of Trichodina Ehrenberg, 1838. Systematic Parasitology, 67, 65–72. https://doi.org/10.1007/s11230-006-9070-6