Abstract
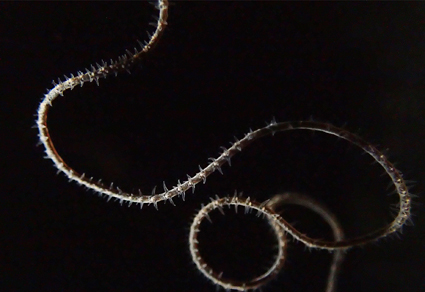
Black corals are key species of marine ecosystems. They can be found in dense aggregations worldwide, but some parts of the world remain totally unexplored. This is the case of the Mesophotic Coral Ecosystem of Mo’orea where the Under the Pole scientific expedition explored mesophotic ecosystems between 60 and 120 m depth and focused on whip black corals. A total of 64 specimens were analyzed morphologically and genetically, and all belonged to the genus Stichopathes. Among them, we describe the new species Stichopathes desaturata sp. nov. It is characterized by an unbranched corallum, irregularly sinuous, with a basal diameter not exceeding 1 mm, reaching a dozen of cm in height. The polyps measure 0.50–1.0 mm in transverse diameter, the interpolypar space is well defined and up to 0.50 mm, with 6–8 polyps per cm. The polypar spines are taller than abpolypar spines, reaching 0.13 mm, perpendicular to the corallum, and conical with a pointed tip, with round and/or elongated papillae on two thirds of the spine. The abpolypar spines are conical to triangular, inclined upwards, with the same ornamentation as the polypar spines. We also identified specimens assigned as Stichopathes cf. contorta and four other putative species. Genetic analyses showed that Mo’orea specimens grouped in three different clades. Analyses of endosymbionts showed that the association with Symbiodiniaceae was likely not involved in the process of host species delineation.
References
- Bo, M., Barucca, M., Biscotti, M.A., Canapa, A., Lapian, H.F.N., Olmo, E. & Bavestrello, G. (2009) Description of Pseudocirrhipathes (Cnidaria: Anthozoa: Hexacorallia: Antipathidae), a new genus of whip black corals from the Indo‐Pacific. Italian Journal of Zoology, 76 (4), 392–402. https://doi.org/10.1080/11250000802684104
- Bo, M., Bavestrello, G., Barucca, M., Makapedua, D.M., Poliseno, A., Forconi, M., Olmo, E. & Canapa, A. (2012) Morphological and molecular characterization of the problematic whip black coral genus Stichopathes (Hexacorallia: Antipatharia) from Indonesia (North Sulawesi, Celebes Sea). Zoological Journal of the Linnean Society, 166 (1), 1–13. https://doi.org/10.1111/j.1096-3642.2012.00834.x
- Bo, M. & Opresko, D.M. (2015) Redescription of Stichopathes pourtalesi Brook, 1889 (Cnidaria: Anthozoa: Antipatharia: Antipathidae). Breviora, 540 (1), 1–18. https://doi.org/10.3099/MCZ16.1
- Bo, M., Montgomery, A.D., Opresko, D.M., Wagner, D. & Bavestrello, G. (2019) Antipatharians of the mesophotic zone: four case studies. . In: Loya, Y., Puglise, K. & Bridge, T. (Eds.), Meophotic Coral Ecosystems. Coral Reefs of the World. Vol. 12. Springer, Cham, pp. 683–708. https://doi.org/10.1007/978-3-319-92735-0_37
- Brugler, M.R., Opresko, D.M. & France, S.C. (2013) The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zoological Journal of the Linnean Society, 169 (2), 312–361. https://doi.org/10.1111/zoj.12060
- Brook, G. (1889) Report on the Antipatharia collected by HMS Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of HMS Challenger During the Years 1873–76, Zoology, 32 (1), 1–222.
- Chimienti, G., De Padova, D., Mossa, M. & Mastrototaro, F. (2020) A mesophotic black coral forest in the Adriatic Sea. Scientific reports, 10 (1), 1–15. https://doi.org/10.1038/s41598-020-65266-9
- Czechowska, K., Feldens, P., Tuya, F., Cosme de Esteban, M., Espino, F., Haroun, R., Schönke, M. & Otero-Ferrer, F. (2020) Testing side-scan sonar and multibeam echosounder to study black coral gardens: a case study from Macaronesia. Remote Sensing, 12 (19), 3244. https://doi.org/10.3390/rs12193244
- Comeau, A.M., Douglas, G.M. & Langille, M.G. (2017) Microbiome helper: a custom and streamlined workflow for microbiome research. MSystems, 2 (1), e00127. https://doi.org/10.1128/mSystems.00127-16
- Cooper, C.F. (1903) Antipatharia. In: Stanley Gardiner, M.A. (Ed.), The Fauna and Geography of the Maldive and Laccadive Archipelagos. Vol. 2. Part 3. Cambridge University Press, Cambridge, pp. 791–796.
- Cooper, C.F. (1909) No. XVII.—Antipatharia. Transactions of the Linnean Society of London, Series 2, Zoology, 12 (4), 301–321. https://doi.org/10.1111/j.1096-3642.1909.tb00144.x
- Cordeiro, R.T.S., Maranhão, H.A., da Silva Lima, S.T. & Pérez, C.D. (2012) First record of Stichopathes occidentalis (Gray, 1860) and range extensions of Antipathes atlantica Gray, 1857 (Cnidaria: Anthozoa: Antipatharia) in the southwestern Atlantic Ocean. Check List, 8 (4), 826–828. https://doi.org/10.15560/8.4.826
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012) jModelTest2: more models, new heuristics and parallel computing. Nature Methods, 9 (8), 772. https://doi.org/10.1038/nmeth.2109
- Flot, J.F. (2007) Champuru 1.0: a computer software for unraveling mixtures of two DNA sequences of unequal lengths. Molecular Ecology Resources, 7 (6), 974–977. https://doi.org/10.1111/j.1471-8286.2007.01857.x
- Flot, J.F. (2010) SeqPHASE: a web tool for interconverting PHASE input/output files and FASTA sequence alignments. Molecular Ecology Resources, 10 (1), 162–166. https://doi.org/10.1111/j.1755-0998.2009.02732.x
- Flot, J.F., Tillier, A., Samadi, S. & Tillier, S. (2006) Phase determination from direct sequencing of length-variable DNA regions. Molecular Ecology Resources, 6 (3), 627–630. https://doi.org/10.1111/j.1471-8286.2006.01355.x
- Flot, J.F., Blanchot, J., Charpy, L., Cruaud, C., Licuanan, W.Y., Nakano, Y., Payri, C. & Tillier, S. (2011) Incongruence between morphotypes and genetically delimited species in the coral genus Stylophora: phenotypic plasticity, morphological convergence, morphological stasis or interspecific hybridization? BMC Ecology, 11 (1), 1–14. https://doi.org/10.1186/1472-6785-11-22
- Fontaneto, D., Flot, J.F. & Tang, C.Q. (2015) Guidelines for DNA taxonomy, with a focus on the meiofauna. Marine Biodiversity, 45 (3), 433–451. https://doi.org/10.1007/s12526-015-0319-7
- Godefroid, M., Hédouin, L., Mercière, A., Under The Pole Consortium & Dubois, P. (2022) Thermal stress responses of the antipatharian Stichopathes sp. from the mesophotic reef of Mo’orea, French Polynesia. Science of The Total Environment, 153094. https://doi.org/10.1016/j.scitotenv.2022.153094
- Godefroid, M., Dubois, P., Under The Pole Consortium & Hédouin, L. (2023) Thermal performance with depth: Comparison of a mesophotic scleractinian and an antipatharian species subjected to internal waves in Mo’orea, French Polynesia. Marine Environmental Research, 184, 105851. https://doi.org/10.1016/j.marenvres.2022.105851
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59 (3), 307–321. https://doi.org/10.1093/sysbio/syq010
- Gress, E., Opresko, D.M., Brugler, M.R., Wagner, D., Eeckhaut, I. & Terrana, L. (2020) Widest geographic distribution of a shallow and mesophotic antipatharian coral (Anthozoa: Hexacorallia): Antipathes grandis VERRILL, 1928–confirmed by morphometric and molecular analyses. Marine Biodiversity Records, 13 (1), 1–7. https://doi.org/10.1186/s41200-020-00195-0
- Gress, E., Eeckhaut, I., Godefroid, M., Dubois, P., Richir, J. & Terrana, L. (2021) Investigation into the presence of Symbiodiniaceae in Antipatharians (Black Corals). Oceans, 2, 772–784. https://doi.org/10.1186/s41200-020-00195-0
- Horowitz, J., Brugler, M.R., Bridge, T.C. & Cowman, P.F. (2020) Morphological and molecular description of a new genus and species of black coral (Cnidaria: Anthozoa: Hexacorallia: Antipatharia: Antipathidae: Blastopathes) from Papua New Guinea. Zootaxa, 4821 (3), 553–569. https://doi.org/10.11646/zootaxa.4821.3.7
- Hume, B.C., D’Angelo, C., Smith, E.G., Stevens, J.R., Burt, J. & Wiedenmann, J. (2015) Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Scientific reports, 5 (1), 1–8. https://doi.org/10.1038/srep08562
- Hunter, C.L., Morden, C.W. & Smith, C.M. (1997) The utility of ITS sequences in assessing relationships among zooxanthellae and corals. Proceedings of the 8th International Coral Reef Symposium, 1997, 1599–1602.
- Jukes, T.H. & Cantor, C.R. (1969) Evolution of protein molecules. In: Munro, H.N. (Ed.), Mammalian Metabolism. Academic Press, New York, New York, pp. 21–132. https://doi.org/10.1016/B978-1-4832-3211-9.50009-7
- Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in bioinformatics, 20 (4), 1160–1166. https://doi.org/10.1093/bib/bbx108
- Lapian, H.F., Barucca, M., Bavestrello, G., Biscotti, M.A., Bo, M., Canapa, A., Tazioli, S. & Olmo, E. (2007) A systematic study of some black coral species (Antipatharia, Hexacorallia) based on rDNA internal transcribed spacers sequences. Marine Biology, 151 (2), 785–792. https://doi.org/10.1007/s00227-006-0525-8
- Letunic, I. & Bork, P. (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Research, 49(W1), W293–W296.Miller, K., Williams, A., Rowden, A. A., Knowles, C., & Dunshea, G. (2010). Conflicting estimates of connectivity among deep‐sea coral populations. Marine Ecology, 31, 144–157. https://doi.org/10.1111/j.1439-0485.2010.00380.x
- Molodtsova, T. & Opresko, D.M. (2022) World List of Antipatharia. Accessed through: World Register of Marine Species. Available from: https://marinespecies.org (accessed 1 July 2024)
- Opresko, D.M. & Sanchez, J.A. (2005) Caribbean shallow-water black corals (Cnidaria: Anthozoa: Antipatharia). Caribbean Journal of Science, 41 (3), 492–507.
- Opresko, D.M., Bo, M., Stein, D.P., Evankow, A., Distel, D.L. & Brugler, M.R. (2021) Description of two new genera and two new species of antipatharian corals in the family Aphanipathidae (Cnidaria: Anthozoa: Antipatharia). Zootaxa, 4966 (2), 161–174. https://doi.org/10.11646/zootaxa.4966.2.4
- Reimer, J.D., Takishita, K., Ono, S. & Maruyama, T. (2007) Diversity and evolution in the zoanthid genus Palythoa (Cnidaria: Hexacorallia) based on nuclear ITS-rDNA. Coral Reefs, 26, 399–410. https://doi.org/10.1007/s00338-007-0210-5
- Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19 (12), 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
- Sánchez, J.A., González-Zapata, F.L., Dueñas, L.F., Andrade, J., Pico-Vargas, A.L., Vergara, D.C., Sarmiento, A. & Bolaños, N. (2019) Corals in the mesophotic zone (40–115 m) at the barrier reef complex from San Andrés Island (southwestern Caribbean). Frontiers in Marine Science, 536. https://doi.org/10.3389/fmars.2019.00536
- Silberfeld, E. (1909) Diagnosen neuer japanischer Antipatharien aus der Sammlung von Herrn Prof Doflein (München). Zoolischer Anzeiger, 34, 760–763.
- Stephens, M., Smith, N.J. & Donnelly, P. (2001) A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics, 68 (4), 978–989. https://doi.org/10.1086/319501
- Summers, S.L. (1910) VIII.—Antipatharians from the Indian Ocean. Journal of the Royal Microscopical Society, 30 (3), 273–281. https://doi.org/10.1111/j.1365-2818.1910.tb01715.x
- Tamura, K., Stecher, G. & Kumar, S. (2021) MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38 (7), 3022–3027. https://doi.org/10.1093/molbev/msab120
- Tazioli, S., Bo, M., Boyer, M., Rotinsulu, H. & Bavestrello, G. (2007) Ecological observations of some common antipatharian corals in the marine park of Bunaken (North Sulawesi, Indonesia). Zoological Studies, 46 (2), 227–241.
- Tapia-Guerra, J.M., Asorey, C.M., Easton, E.E., Wagner, D., Gorny, M. & Sellanes, J. (2021) First Ecological Characterization of Whip Black Coral Assemblages (Hexacorallia: Antipatharia) in the Easter Island Ecoregion, Southeastern Pacific. Frontiers in Marine Science, 8, 755898. https://doi.org/10.3389/fmars.2021.755898
- Terrana, L., Bo, M., Opresko, D.M. & Eeckhaut, I. (2020) Shallow-water black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia) from SW Madagascar. Zootaxa, 4826 (1), 1–62. https://doi.org/10.11646/zootaxa.4826.1.1
- Terrana, L., Flot, J.F. & Eeckhaut, I. (2021) ITS1 variation among Stichopathes cf. maldivensis (Hexacorallia: Antipatharia) whip black corals unveils conspecificity and population connectivity at local and global scales across the Indo-Pacific. Coral Reefs, 40 (2), 521–533. https://doi.org/10.1007/s00338-020-02049-8
- Thomson, J.A. & Simpson, J.J. (1905) Report of the Antipatharia collected by Prof. Herdman at Ceylon, 1902. Report to the government of Ceylon on the pearl oyster fisheries of the gulf of Manaar, 4, 93–106.
- Van Pesch, A.J. (1914) The Antipatharia of the Siboga Expedition. Siboga-Expeditie Monographes, 17, 1–258. https://doi.org/10.5962/t.173163
- Wagner, D., Brugler, M.R., Opresko, D.M., France, S.C., Montgomery, A.D. & Toonen, R.J. (2010) Using morphometrics, in situ observations and genetic characters to distinguish among commercially valuable Hawaiian black coral species; a redescription of Antipathes grandis Verrill, 1928 (Antipatharia: Antipathidae). Invertebrate Systematics, 24 (3), 271–290. https://doi.org/10.1071/IS10004
- Wagner, D., Papastamatiou, Y.P., Kosaki, R.K., Gleason, K.A., McFall, G.B., Boland, R.C., Pyle, R.L. & Toonen, R.J. (2011) New Records of Commercially Valuable Black Corals (Cnidaria: Antipatharia) from the Northwestern Hawaiian Islands at Mesophotic Depths. Pacific Science, 65 (2), 249–255. https://doi.org/10.2984/65.2.249
- Wagner, D., Luck, D.G. & Toonen, R.J. (2012) The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Advances in Marine Biology, 63, 67–132. https://doi.org/10.1016/B978-0-12-394282-1.00002-8