Abstract
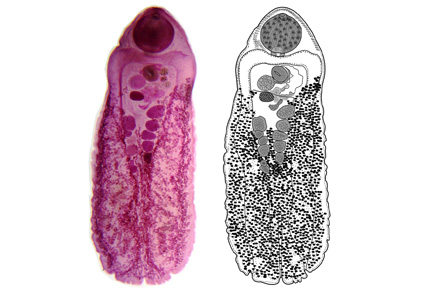
The morphology and host-parasite data of Schistorchis carneus Lühe, 1906 indicate low host specificity and geographical range variation, accompanied by a large scale of intraspecific variability and ambiguous molecular phylogeny, confusing in determining biodiversity extent and specificity to a particular host and/or locality. To address the challenging circumstances of records of S. carneus, a detailed morphological description, molecular characterization and species delimitation analyses were conducted using a combination of comparative morphology, molecular phylogeny, multivariate analyses, and host-parasite data. Several morphological features exhibited an extensive intra-variation, as well as a wide morphometric range in many measurements within and across records. Phylogenetic analyses showed that the classification of schistorchiines is highly correlated with the morphology/nature of the oral sucker and highlighted the limitations of ribosomal 28S rDNA and ITS2 rDNA in distinguishing morphologically close/related taxa. Species delimitation analyses reflected the critical importance of host distinction in schistorchiines recognition/differentiation, even those that are indistinct morphologically. The clustering of schistorchiines into certain groups was driven by host differences. Additionally, host variation typically corresponds to a distinct schistorchiine species, even if it is morphologically identical to another. Cluster analysis associated with host-parasite data revealed high morphometric convergence and significant diversity among Egyptian and Sri-Lankan records of S. carneus. It also confirmed the distinction between Indian records from the Bay of Bengal and those from the Gulf of Mannar (previously mentioned as Manaar), each in their own distant group. In conclusion, the records of S. carneus represent a repository for a group of closely related cryptic species. The restricted concept of S. carneus should include only records from the white-spotted puffer, Arothron hispidus (Linnaeus) and the stellate puffer, Arothron stellatus (Anonymous). The Australian specimens from the narrow-lined puffer, Arothron manilensis (Marion de Procé) and the Indian record from the lunartail puffer, Lagocephalus lunaris (Bloch & Schneider) likely represent a free-standing cryptic species with limited distribution, requiring further characterization.
References
- Abid-Kachour, S., Mouffok, S. & Boutiba, Z. (2013) Description of a New Species of Sphincteristomum from Sparid Fishies of the Algerian Coast (Western Mediterranean). Journal of Environmental Protection, 4, 1129–1136. https://doi.org/10.4236/jep.2013.410129
- Anderson, R.C. & Barker S.C. (1998) Inference of phylogeny and taxonomy within the Didymozoidae (Digenea) from the second internal transcribed spacer (ITS2) of ribosomal DNA. Systematic Parasitology, 41, 87–94. https://doi.org/10.1023/a:1006024128098
- Andres, M.J., Pulis, E.E., Curran, S.S. & Overstreet, R.M. (2018) On the systematics of some marine haploporids (Trematoda) with the description of a new species of Megasolena Linton, 1910. Parasitology International, 67, 805–815. https://doi.org/10.1016/j.parint.2018.08.002
- Anter, R., Georgieva, S., Gargouri, L. & Kostadinova, A. (2015) Molecular evidence for the existence of species complexes within Macvicaria Gibson & Bray, 1982 (Digenea: Opecoelidae) in the western Mediterranean, with descriptions of two new species. Systematic Parasitology, 91, 211–229. https://doi.org/10.1007/s11230-015-9577-9
- Ashour, A.A. (1995) Scanning electron microscope observations on Corrigia vitta (Dujardin, 1845) Shtrom, 1940 (Trematoda: Dicrocoeliidae). Journal of the Egyptian Society of Parasitology, 25 (1), 25–30.
- Bennett, C.E. (1975) Scanning electron microscopy of Fasciola hepatica L. during growth and maturation in the mouse. The Journal of Parasitology, 61, 892–898. https://doi.org/10.2307/3279230
- Blasco-Costa, I., Cutmore, S.C., Miller, T.L. & Nolan, M.J. (2016) Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Systematic Parasitology, 93, 295–306. https://doi.org/10.1007/s11230-016-9631-2
- Blend, C.K., Karar, Y.F.M. & Dronen, N.O. (2017) Revision of the Megaperidae Manter, 1934 n. comb. (Syn. Apocreadiidae Skrjabin, 1942) including a reorganization of the Schistorchiinae Yamaguti, 1942. Zootaxa, 4358 (1), 1–44. https://doi.org/10.11646/zootaxa.4358.1.1
- Blend, C.K., Karar, Y.F. & Dronen, N.O. (2024) Decision favoring Megaperidae Manter, 1934 and errata as relates to Blend et al. 2017. Zootaxa, 5424 (1), 136–138. https://doi.org/10.11646/zootaxa.5424.1.8
- Bray, R.A. (2005a) Superfamily Lepocreadioidea Odhner, 1905. In: Jones, A., Bray, R.A. & Gibson, D.I. (Eds.), Keys to the Trematoda. Vol. 2. CABI Publishing and the Natural History Museum, Wallingford, pp. 541–543. https://doi.org/10.1079/9780851995878.0541
- Bray, R.A. (2005b) Family Megaperidae Manter, 1934. In: Jones, A., Bray, R.A. & Gibson, D.I. (Eds.), Keys to the Trematoda. Vol. 2. CABI Publishing and the Natural History Museum, Wallingford, pp. 683–685. https://doi.org/10.1079/9780851995878.0683
- Bray, R.A., Cutmore, S.C. & Cribb, T.H. (2022) A paradigm for the recognition of cryptic trematode species in tropical Indo-west Pacific fishes: the problematic genus Preptetos (Trematoda: Lepocreadiidae). International Journal for Parasitology, 52, 169–203. https://doi.org/10.1016/j.ijpara.2021.08.004
- Bray, R.A., Waeschenbach, A., Cribb, T.H., Weedall, G.D., Dyal, P. & Littlewood, D.T.J. (2009) The phylogeny of the Lepocreadioidea (Platyhelminthes, Digenea) inferred from nuclear and mitochondrial genes: Implications for their systematics and evolution. Acta Parasitologica, 54, 310–329. https://doi.org/10.2478/s11686-009-0045-z
- Bush, A.O., Lafferty, K.D., Lotz, J.M. & Shostak, A.W. (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83, 575–583. https://doi.org/10.2307/3284227
- Ceccolini, F. & Cianferoni, F. (2021) Nomenclature changes in the flatworms (Platyhelminthes). Invertebrate Zoology, 18, 451–456. https://doi.org/10.15298/invertzool.18.4.02
- Cribb, T.H. (2005) Family Apocreadiidae Skrjabin, 1942. In: Jones, A., Bray, R.A. & Gibson, D.I. (Eds.), Keys to the Trematoda. Vol. 2. CABI Publishing and the Natural History Museum, Wallingford, pp. 621–639. https://doi.org/10.1079/9780851995878.0621
- Cribb, T.H. & Bray, R.A. (1999) A review of the Apocreadiidae Skrjabin, 1942 (Trematoda: Digenea) and description of Australian species. Systematic Parasitology, 44, 1–36. https://doi.org/10.1023/a:1006197201426
- Cribb, T.H. & Bray, R.A. (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7. https://doi.org/10.1007/s11230-010-9229-z
- Cribb, T.H., Adlard, R.D. & Bray, R.A. (1998) A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). International Journal for Parasitology, 28, 1791−1795. https://doi.org/10.1016/s0020-7519(98)00127-1
- Curran, S.S., Tkach, V.V. & Overstreet, R.M. (2013) Molecular evidence for two cryptic species of Homalometron (Digenea: Apocreadiidae) in freshwater fishes of the southeastern United States. Comparative Parasitology, 80, 186–195. https://doi.org/10.1654/4626.1
- De León, G.P.P. & Hernández-Mena, D. (2019) Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. Journal of Helminthology, 93, 260–276. https://doi.org/10.1017/s0022149x19000191
- De León, G. Pérez-Ponce, Razo-Mendivil, U. & Garcia-Magana, L. (2012) Morphological and molecular evidences for the existence of two new species of Homalometron (Digenea: Apocreadiidae), parasites of cichlids (Osteichthyes: Cichlidae). Zootaxa, 3407 (1), 37–48. https://doi.org/10.11646/zootaxa.3407.1.3
- Durio, W.O. & Manter, H.W. (1969) Some digenetic trematodes of marine fishes of New Caledonia. III. Acanthocolpidae, Haploporidae, Gyliauchenidae, and Cryptogonimidae. Journal of Parasitology, 55, 293–300. https://doi.org/10.2307/3277393
- Edler, D., Klein, J., Antonelli, A. & Silvestro, D. (2021) raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution, 12, 373–377. https://doi.org/10.1111/2041-210X.13512
- Fayton, T.J., Curran, S.S., Andres, M.J., Overstreet, R.M. & McAllister, C.T. (2016) Two new species of Homalometron (Digenea: Apocreadiidae) from Nearctic freshwater fundulids, elucidation of the life cycle of H. cupuloris, and molecular phylogenetic analysis of some congeners. The Journal of Parasitology, 102, 94–104. https://doi.org/10.1645/15-862
- Froese, R. & Pauly, D. (Eds.) (2024). FishBase. Version 06/2024. http://www.fishbase.org (accessed 5 November 2024)
- Gomes, F.P. (1985) Curso de estatística experimental. Nobel, São Paulo, 467 pp.
- Hafeezullah, M. (1981) Schistorchiid trematodes of marine fishes of India, with considerations on the status of the genus Megacreadium Nagaty, 1956 and Family Schistorchiidae Yamaguti, 1942. Bulletin of the Zoological Survey of India, 3, 167–177.
- Hanson, M.L. (1953) A discussion of the trematode genus Schistorchis (Family Lepocreadiidae) with descriptions of two new species from Hawaii. Pacific Science, 7, 447–452.
- Hassan, F.A., Tolba, M.E.M., Abed, G.H., Omar, H.M. & Abdel-Hakeem, S.S. (2021) Contact lenses contamination by Acanthamoeba spp. in Upper Egypt. Plos One, 16, e0259847. https://doi.org/10.1371/journal.pone.0259847
- Hershkovitz, M.A. & Lewis, L.A. (1996) Deep-level diagnostic value of the rDNA-ITS region. Molecular biology and evolution, 13, 1276–1295. https://doi.org/10.1093/oxfordjournals.molbev.a025693
- Hernández-Mena, D.I., Cabañas-Granillo, J., Medina-Hernández, E. & de León, G.P.P. (2022) Discovery of a new species of Homalometron Stafford, 1904 (Digenea: Apocreadiidae) from the striped mojarra, Eugerres plumieri in a coastal lagoon of the Gulf of Mexico. Journal of Helminthology, 96, e46. https://doi.org/10.1017/s0022149x22000372
- Hoole, D. & Mitchell, J.B. (1981) Ultrastructural observations on the sensory papillae of juvenile and adult Gorgoderina vitelliloba (Trematoda: Gorgoderidae). International Journal for Parasitology, 11, 411–417. https://doi.org/10.1016/0020-7519(81)90014-x
- Huston, D.C., Cutmore, S.C. & Cribb, T.H. (2017) Isorchis cannoni n. sp. (Digenea: Atractotrematidae) from Great Barrier Reef rabbitfishes and the molecular elucidation of its life cycle. Journal of Helminthology, 92, 604–611. https://doi.org/10.1017/s0022149x17000906
- ICZN (2022) Opinion 2483 (Case 3775) – MEGAPERIDAE Manter, 1934 (Platyhelminthes, Digenea): precedence re-established over APOCREADIINAE Skrjabin, 1942. Bulletin of Zoological Nomenclature, 79, 61–62. https://doi.org/10.21805/bzn.v79.a013
- Johnston, S.J. (1913) On some Queensland trematodes, with anatomical observations and descriptions of new species and genera. Quarterly Journal of Microscopical Science, 59, 361–400. https://doi.org/10.1242/jcs.s2-59.235.361
- Jones, A. (2005) Introduction and key to superfamilies. In: Jones, A., Bray, R.A. & Gibson, D.I. (Eds.), Keys to the Trematoda. Vol. 2. CABI Publishing and the Natural History Museum, Wallingford, pp. 1–4. https://doi.org/10.1079/9780851995878.0001
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/ 10.1093/molbev/msy096
- Kuzmin, Y.I., Halajian, A., Tavakol, S., Luus-Powell, W.J. & Tkach, V.V. (2017) Description and phylogenetic position of a new species of Rhabdias Stiles et Hassall, 1905 (Nematoda: Rhabdiasidae) from the banded rubber frog, Phrynomantis bifasciatus (Smith) (Amphibia: Microhylidae), in South Africa. Folia Parasitologica, 64, 1–8. https://doi.org/10.14411/fp.2017.035
- Lehman, A. (2005) JMP for basic univariate and multivariate statistics: a step-by-step guide. , SAS Institute Inc., Cary, North Carolina, 558 pp.
- Lewis, P.O., Holder, M.T. & Holsinger, K.E. (2005) Polytomies and Bayesian phylogenetic inference. Systematic Biology, 54, 241–253. https://doi.org/10.1080/10635150590924208
- Lieske, E., Fiedler, K.E. & Myers, R.F. (2004) Coral Reef Guide: Red Sea to Gulf of Aden, South Oman; [the definitive guide to over 1200 species of underwater life]. Collins, London, 384 pp. https://doi.org/10.5860/choice.42-5255
- Linton, E.W. (1907) Notes on parasites of Bermuda fishes. Proceedings of the U.S. National Museum, 33, 85–126. https://doi.org/10.5479/si.00963801.33-1560.85
- Lo, C.M., Morgan, J.A., Galzin, R. & Cribb, T.H. (2001) Identical digeneans in coral reef fishes from French Polynesia and the Great Barrier Reef (Australia) demonstrated by morphology and molecules. International Journal for Parasitology, 31, 1573–1578. https://doi.org/10.1016/s0020-7519(01)00306-x
- Lühe, M.F.L. (1906) Report on the trematode parasites from the marine fishes of Ceylon. The Royal Society Report on the Pearl Oyster Fisheries, 5, 97–108.
- MacCallum, W.G. (1895) On the anatomy of two Distoma parasites of freshwater fish. Veterinary Magazine Philadelphia, 2, 401–412.
- Machida, M. & Kuramochi, T. (1999) Digenean trematodes from Tetraodontiform fishes from Japanese and adjacent waters. Bulletin of the National Science Museum. Series A, Zoology, 25, 1–25.
- Madhavi, R. & Bray, R.A. (2018) Digenetic Trematodes of Indian Marine Fishes. Springer, Dordrecht, 693 pp. https://doi.org/10.1007/978-94-024-1535-3
- Madhavi, R., Narasimhulu, S.V. & Shameem, U. (1986) Digenetic trematodes from marine fishes of Kalingapatnam coast, Bay of Bengal. Families Lepocreadiidae, Deropristiidae and Schistorchiidae. Rivista di Parassitologia, 47, 111–119.
- Magro, L., Cutmore, S.C., Carrasson, M. & Cribb, T.H. (2023) Integrated characterisation of nine species of the Schistorchiinae (Trematoda: Apocreadiidae) from Indo-Pacific fishes: two new species, a new genus, and a resurrected but ‘cryptic’genus. Systematic Parasitology, 100, 381–413. https://doi.org/10.1007/s11230-023-10093-5
- Manter, H.W. (1933) A new family of trematodes from marine fishes. Transactions of the American Microscopical Society, 52, 233–242. https://doi.org/10.2307/3222258
- Manter, H.W. (1934) Megapera, new name for the trematode genus Eurypera Manter, 1933. Transactions of the American Microscopical Society, 53, 293. https://doi.org/10.2307/3222107
- Manter, H.W. (1936) Some trematodes of cenote fish from Yucatan. Publications of the Carnegie Institution of Washington, 457, 33–38.
- Manter, H.W. (1947) The digenetic trematodes of marine fishes of Tortugas, Florida. American Midland Naturalist, 38, 257–416. https://doi.org/10.2307/2421571
- Manter, H.W. (1963). Studies on digenetic trematodes of fishes of Fiji. II. Families Lepocreadiidae, Opistholebetidae, and Opecoelidae. Journal of Parasitology, 49, 99–113.
- Martorelli, S.R. (1986) Estudios parasitológicos en biotopos lénticos de la República Argentina II: El ciclo biológico de Homalometron pseudopallidum sp. nov. (Digenea) parásito de Gymnogeophagus australis Eigenman, 1907 (Pisces, Cichlidae). Neotropica, 32, 3–12.
- Nagaty, H.F. (1957) Trematodes of fishes from the Red Sea. Part 8. Five species in the families Schistorchidae, Acanthocolpidae, and Heterophyidae. The Journal of parasitology, 43, 217−220. https://doi.org/10.2307/3274653
- Nollen, P.M., Restaino, A.L. & Alberico, R.A. (1973) Gorgoderina attenuata: Uptake and incorporation of tyrosine, thymidine, and adenosine. Experimental Parasitology, 33, 468−476. https://doi.org/10.1016/0014-4894(73)90114-8
- Nylander, J.A.A. (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. https://doi.org/10.1186/1471-2148-4-23
- Odhner, T. (1928) Weitere Trematoden mit anus. Akriv för Zoologi, 20, 1–6.
- Olson, P.D., Cribb, T.H., Tkach, V.V., Bray, R.A. & Littlewood, D.T.J. (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755. https://doi.org/10.1016/s0020-7519(03)00049-3
- Oshmarin, P.G., Mamaev, Yu.L. & Parukhin, A.M. (1961) New species of trematodes of the family Diploproctodaeidae Ozaki, 1928. Helminthologia, 3, 254–260.
- Overstreet, R.M. (1970) Two new species of Digenea from the spot, Leiostomus xanthurus Lacépède, from the Gulf of Mexico. Journal of Parasitology, 56, 1055–1057. https://doi.org/10.2307/3277546
- Parukhin, A.M. (1963) New species of trematodes from fish of the South China Sea. In: Skjrabin, K.I. (Ed.), Helminths of Man, Animals and Plants and their control: Papers on helminthology presented to Academician K.I. Skrjabin on his 85th birthday. Izdatelstov Akadémie Nauk, SSSR, Moscow, pp. 123–125. [in Russian]
- Pantoja, C., Scholz, T., Luque, J.L. & Perez-Ponce de León, G. (2021) Molecular and morphological evidence of a new species of Crassicutis Manter, 1936 (Digenea), a parasite of cichlids in South America. Parasitology Research, 120, 2429–2443. https://doi.org/10.1007/s00436-021-07161-4
- Panyarachun, B., Sobhon, P., Tinikul, Y., Chotwiwatthanakun, C., Anupunpisit, V. & Anuracpreeda, P. (2010) Paramphistomum cervi: surface topography of the tegument of adult fluke. Experimental Parasitology, 125, 95–99.
- Parkening, A.T. & Otubanjo, O.A. (1985) Scanning electron microscopic studies of the body surface and external genitalia of a dicrocoeliid trematode, Concinnum epomopis Sandground 1973. Zeitschrift für Parasitenkunde, 71, 495–504.
- Parkening, A.T & Johnson, A.D. (1969). Glucose uptake in Haematoloechus medioplexus and Gorgoderina trematodes. Experimental Parasitology, 25, 358–367. https://doi.org/10.1016/0014-4894(69)90082-4
- Parker, J. H., Curran, S.S., Overstreet, R.M. & Tkach, V.V. (2010) Examination of Homalometron elongatum Manter, 1947 and description of a new congener from Eucinostomus currani Zahuranec, 1980 in the Pacific Ocean off Costa Rica. Comparative Parasitology, 77, 154–163. https://doi.org/10.1654/4451.1
- Price, E.W. (1932) The trematode parasites of marine mammals. Proceedings of the United States National Museum, 81, 1–68. https://doi.org/10.5479/si.00963801.81-2936.1
- Pritchard, M.H. (1963) Studies on Digenetic Trematodes of Hawaiian Fishes, Primarily Families Lepocreadiidae and Zoogonidae. Journal of Parasitology. 49, 578–587. https://doi.org/10.2307/3275763
- Pulis, E.E., Curran, S.S., Andres, M.J. & Overstreet, R.M. (2014) Change in rank of Megaperidae (Trematoda) to Megaperinae within the Apocreadiidae and description of Haintestinum amplum n. g., n. sp. Parasitology International, 63, 269–274. https://doi.org/10.1016/j.parint.2013.11.007
- Rambaut, A. (2018) FigTree v.1.4.4. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 5 November 2024)
- Ramsey, J.S. (1965). Barbulostomum cupuloris gen. et sp. n. (Trematoda: Lepocreadiidae) from sunfishes (Lepomis spp.) in Lake Pontchartrain, Louisiana. Journal of Parasitology, 51,777–780. https://doi.org/10.2307/3276157
- Randall, J.E. (1982) The Diver's Guide to Red Sea Reef Fishes. IMMEL Publishing, University of California, 96 pp.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Siddiqi, A.H. & Cable, R.M (1960). Digenetic trematodes of marine fishes of Puerto Rico. Scientific Survey of Porto Rico and the Virgin Islands, 17, 257–369. [https://babel.hathitrust.org/cgi/pt?id=mdp.39015069708819;view=1up;seq=1]
- Skrjabin, K.I. (1959) Family Schistorchidae Yamaguti, 1942. Osnovy Trematodologii, 16, 14–51. [in Russian]
- Stafford, J. (1904) Trematodes from Canadian fishes. Zoologischer Anzeiger, 27, 481–495.
- Swarnakar, G., Kumawat, A., Sanger, B., Roat, K., Goswami, H. & Damor, R.N. (2014) Scanning electron microscopic observations of rumen amphistome (Trematoda) in buffalo (Bubalus bubalis) in Udaipur, Rajasthan. International Journal of Current Microbiology and Applied Sciences, 3, 695–703.
- Tkach, V.V., Littlewood, D.T.J., Olson, P.D., Kinsella, J.M. & Swiderski, Z. (2003) Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Systematic Parasitology, 56, 1–15. https://doi.org/10.1023/a:1025546001611
- Toman, G. (1989) Digenetic trematodes of marine teleost fishes from the Seychelles, Indian Ocean. II. Acta Parasitologica Polonica, 34, 235–246.
- WoRMS (2024a) Megaperidae Manter, 1934. World Register of Marine Species (WoRMS). Available from: https://www.marinespecies.org/aphia.php?p=taxdetails&id=414843 (accessed 5 November 2024)
- WoRMS (2024b) Schistorchiinae Yamaguti, 1942. World Register of Marine Species (WoRMS). Available from: https://www.marinespecies.org/aphia.php?p=taxdetails&id=734041 (accessed 5 November 2024)
- Yamaguti, S. (1942). Studies on the helminth fauna of Japan. Part 39. Trematodes of fishes mainly from Naha. Transactions of the Biogeographical Society of Japan, 3, 329–398.
- Yamaguti, S. (1953). Parasitic worms mainly from Celebes. Part 3. Digenetic trematodes of fishes. II. Acta Medicinae Okayama, 8, 257–295.
- Yamaguti, S. (1958) Systema Helminthum. Vol 1. The Digenetic Trematodes of Vertebrates. Part 1. Interscience Publishers, New York, 979 pp.
- Yamaguti, S. (1970) Digenetic Trematodes of Hawaiian Fishes. Keigaku Publishing Company, Tokyo, 436 pp.
- Yamaguti, S. (1971) Synopsis of Digenetic Trematodes of Vertebrates. Vol. 1. Keigaku Publishing Company, Tokyo, 1074 pp.