Abstract
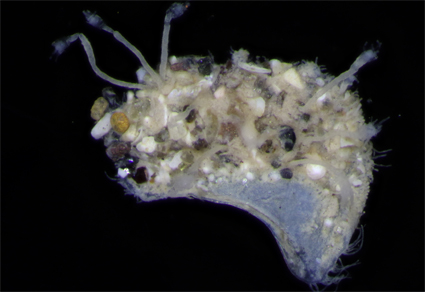
The scaleworm Pelogenia fimbriata (Hartman, 1939) collected from Secas Islands, Panama, during the Allan Hancock Pacific Expedition in 1916, was not only recognized as an undescribed species at the time of discovery but has also revealed a new finding almost nine decades after its description. A paratype of P. fimbriata was examined in the Natural History Museum of Los Angeles County, USA and the hydroid Bimeria vestita Wright, 1959 was found attached to an anterior scale of the worm. The interaction between B. vestita and P. fimbriata in this study represents the first record of epibiosis of a hydroid on a sigalionid scaleworm. This discovery contributes to our understanding of epibiosis in Hydrozoa and opens new avenues for further research in this area.
References
- Barnich, R., Beuck, L. & Freiwald, A. (2013) Scale worms (Polychaeta: Aphroditiformia) associated with cold-water corals in the eastern Gulf of Mexico. Journal of Marine Biological Association of the United Kingdom, 93 (8), 2129–2143. https://doi.org/10.1017/S002531541300088X
- Beckett, A. & Read, N.D. (1986) Low-Temperature Scanning Electron Microscopy. In: Aldrich, H.C. & Todd, W.J. (Eds.), Ultrastructure Techniques for Microorganisms. Springer, Boston, MA., pp. 45–86. https://doi.org/10.1007/978-1-4684-5119-1_2
- Britayev, T.A. & Lyskin, S.A. (2002) Feeding of the symbiotic polychaete Gastrolepidia clavigera (Polynoidae) and its interactions with its hosts. Doklady Biological Sciences, 385 (1/6), 352–356. https://doi.org/10.1023/a:1019964918471
- Britayev, T.A., Krylova, E.M., Martin, D., von Cosel, R. & Aksiuk, T.S. (2003) Symbiont – host interaction in the association of the scaleworm Branchipolynoe aff. seepensis (Polychaeta: Polynoidae) with the hydrothermal mussel Bathymodiolus spp. (Bivalvia: Mytilidae). InterRidge News, 12, 13–16.
- Calder, D.R. (1976) The zonation of hydroids along salinity gradients in South Carolina estuaries. In: Mackie, G.O. (Ed.), Coelenterate ecology and behavior. Plenum Press, New York, New York, pp. 165–174. https://doi.org/10.1007/978-1-4757-9724-4_18
- Calder, D.R. (1988) Shallow-water hydroids of Bermuda: the athecatae. Royal Ontario Museum Life Sciences Contributions, 148, 1–107.
- Calder, D.R. (2010) Some anthoathecate hydroids and limnopolyps (Cnidaria, Hydrozoa) from the Hawaiian archipelago. Zootaxa, 2590 (1), 1–91. https://doi.org/10.11646/zootaxa.2590.1.1
- Calder, D.R. (2013) Some shallow-water hydroids (Cnidaria: Hydrozoa) from the central east coast of Florida, USA. Zootaxa, 3648 (1), 1–72. https://doi.org/10.11646/zootaxa.3648.1.1
- Calder, D.R. (2019) On a collection of hydroids (Cnidaria, Hydrozoa) from the southwest coast of Florida, USA. Zootaxa, 4689 (1), 1–141. https://doi.org/10.11646/zootaxa.4689.1.1
- Calder, D.R., Carlton, J.T., Keith, I., Ashton, G.V., Larson, K., Ruiz, G.M., Herrera, E. & Golfin, G. (2022) Biofouling hydroids (Cnidaria: Hydrozoa) from a Tropical Eastern Pacific island, with remarks on their biogeography. Journal of Natural History, 56 (9–12), 565–606. https://doi.org/10.1080/00222933.2022.2068387
- Cruz-Gómez, C. (2022) Pelogeniinae Chamberlin, 1919 (Annelida, Sigalionidae) from the Grand Caribbean Region. European Journal of Taxonomy, 807, 1–59. https://doi.org/10.5852/ejt.2022.807.1717
- De Assis, J.E., de Souza, J.R.B., de Lima, M.M., de Lima, G.V., Cordeiro, R.T.S. & Pérez, C.D. (2019) Association between deep-water scale-worms (Annelida: Polynoidae) and black corals (Cnidaria: Antipatharia) in the Southwestern Atlantic. Zoologia, 36, 1–13. https://doi.org/10.3897/zoologia.36.e28714
- Di Camillo, C.G., Martin, D. & Britayev, T.A. (2011) Symbiotic association between Solanderia secunda (Cnidaria, Hydrozoa, Solanderiidae) and Medioantenna variopinta sp. nov. (Annelida, Polychaeta, Polynoidae) from North Sulawesi (Indonesia). Helgoland Marine Research, 65, 495–511. https://doi.org/10.1007/s10152-010-0239-7
- Dziubinska, A. & Sapota, M. (2013) Hydroid Gonothyraea loveni found on the straightnose pipefish (Nerophis ophidion) in the Gulf of Gdansk-symbiosis, parasitism or biofouling. Oceanological and Hydrobiological Studies, 42, 332. https://doi.org/10.2478/s13545-013-0090-y
- Eibye-Jacobsen, D., Aungtonya, A. & Gonzalez, B.C. (2022) Sigalionidae Kinberg, 1856. In: Purschke, G., Böggemann, M. & Westheide, W. (Eds.), Pleistoannelida, Errantia II. Vol. 4. Walter de Gruyter GmbH & Co. KG, Berlin, pp. 114–138. https://doi.org/10.1515/9783110647167-006
- Fassio, G. (2024) Coriocella and the Worms: First record of scale-worm Asterophilia cf. culcitae ectosymbiotic on a mollusc. Diversity, 16 (1), 65. https://doi.org/10.3390/d16010065
- Fernandez-Leborans, G., Román, S. & Martin, D. (2017) A new deep-sea suctorian-nematode epibiosis (Loricophrya-Tricoma) from the Blanes submarine canyon (NW Mediterranean). Microbial Ecology, 74, 15–21. https://doi.org/10.1007/s00248-016-0923-5
- Fraser, C.M. (1938a) Hydroids of the 1934 Allan Hancock Pacific Expedition. Allan Hancock Pacific Expeditions, 4, 1–105.
- Fraser, C.M. (1938b) Hydroids of the 1932, 1933, 1935, and 1938 Allan Hancock Pacific Expeditions. Allan Hancock Pacific Expeditions, 4, 129–153.
- Fraser, M.C. (1943) General account of the scientific work of the Velero III in the Eastern Pacific, 1931–41. Allan Hancock Pacific Expeditions, 1 (3), 259–425.
- Genzano, G.N. & Zamponi, M.O. (1999) Natural history of Bimeria vestita Wright, 1859 (Hydrozoa, Bougainvillidae) in the rocky intertidal of Mar del Plata (Argentina). Ciencias Marinas, 25 (1), 63–74. https://doi.org/10.7773/cm.v25i1.652
- Gili, J.M., Murillo, J. & Ros, J. (1989) The distribution pattern of benthic cnidarians in the Western Mediterranean. Scientia Marina, 53, 19–35.
- Gili, J.M. & Hughes, R.G. (1995) The ecology of marine benthic hydroids. Oceanography and marine biology: an annual review, 33, 351–426.
- Goto, R. & Tanaka, M. (2019) Worm-riding clam: description of Montacutona sigalionidcola sp. nov. (Bivalvia: Heterodonta: Galeommatidae) from Japan and its phylogenetic position. Zootaxa, 4652 (3), 473–486. https://doi.org/10.11646/zootaxa.4652.3.4
- Hartman, O. (1939) Polychaetous annelids, 1: Aphroditidae to Pisionidae. Allan Hancock Pacific Expeditions, 7 (1), 1–156.
- Hartmann-Schröder, G. (1992) Drei neue Polychaeten-arten der familien Polynoidae und Syllidae von Neu-Kalendonien, assoziiert mit einer verkalten Hydrozoe. Helgoländer Meeresuntersuchungen, 43, 93–101. https://doi.org/10.1007/BF02366214
- Jumars, P.A., Dorgan, K.M. & Lindsay, S.M. (2015) Diet of worms emended: an updated of polychaete feeding guilds. Annual Review of Marine Science, 7, 497–520. https://doi.org/10.1146/annurev-marine-010814-020007
- Maggioni, D., Schuchert, P., Ostrovsky, A.N., Schiavo, A., Hoeksema, B.W., Pica, D., Piraino, S., Arrigoni, R., Seveso, D., Montalbetti, E., Galli, P. & Montano, S. (2024) Systematics and character evolution of capitate hydrozoans. Cladistics, 40 (2), 107–134. https://doi.org/10.1111/cla.12567
- Martin, D. & Britayev, T.A. (1998) Symbiotic polychaetes: review of known species. Oceanography and Marine Biology: An Annual Review, 36, 217–340.
- Martin, D. & Britayev, T.A. (2018) Symbiotic polychaetes revisited: an update of the known species and relationships (1998–2017). Oceanography and Marine Biology: An Annual Review, 56, 371–448. https://doi.org/10.1201/9780429454455-6
- Mendoza-Becerril, M.A., Marian, J.E.A.R., Migotto, A.E. & Marques, A.C. (2017) Exoskeletons of Bougainvilliidae and other Hydroidolina (Cnidaria, Hydrozoa): structure and composition. PeerJ, 5, e2964. https://doi.org/10.7717/peerj.2964
- Mendoza-Becerril, M.A., Simões, N. & Genzano, G. (2018) Benthic hydroids (Cnidaria, Hydrozoa) from Alacranes Reef, Gulf of Mexico, Mexico. Bulletin of Marine Science, 94 (1), 1–18. https://doi.org/10.5343/bms.2017.1072
- Mendoza-Becerril, M.A., Estrada-Gonzalez, M.C., Mazariegos-Villarreal, A., Restrepo-Avedaño, L., Villar-Beltrán, R.D., Agüero, J. & Cunha, A.F. (2020) Taxonomy and diversity of Hydrozoa (Cnidaria, Medusozoa) of La Paz Bay, Gulf of California. Zootaxa, 4808 (1), 1–37. https://doi.org/10.11646/zootaxa.4808.1.1
- Mikac, B., Semprucci, F., Guidi, L., Ponti, M., Abbiati, M., Balsamo, M. & Dovgal, I. (2020) Newly discovered associations between peritrich ciliates (Ciliophora: Peritrichia) and scale polychaetes (Annelida: Polynoidae and Sigalionidae) with a review of polychaete–peritrich epibiosis. Zoological Journal of the Linnean Society, 188, 939–953.
- Molodtsova, T.N., Britayev, T.A. & Martin, D. (2016) Cnidarians and their Polychaete symbionts. In: Goffredo, S. & Dubinsky, Z. (Eds.), The Cnidaria, Past, Present and Future. Springer, Cham, pp. 387–413. https://doi.org/10.1007/978-3-319-31305-4_25
- Montano, S., Fattorini, S., Parravicini, V., Berumen, M.L., Galli, P., Maggioni, D., Arrigoni, R., Seveso, D. & Strona, G. (2017) Corals hosting symbiotic hydrozoans are less susceptible to predation and disease. Proceedings of the Royal Society B: Biological Sciences, 284 (1869), 20172405. https://doi.org/10.1098/rspb.2017.2405
- Monti, M., Giorgi, A. & Olson, J.B. (2018) Hydroids on a Caribbean sea horse. Coral Reefs, 37, 1085. https://doi.org/10.1007/s00338-018-1725-7
- Nishi, E. & Tachikawa, H. (1999) New record of a commensal scale worm Medioantenna lavate Imajima, 1997 (Polychaeta: Polynoidae), from Ogasawara Islands, Japan. Natural History Research, 5, 107–110.
- Osman, R.W. & Haugsness, J.A. (1981) Mutualism among sessile invertebrates: a mediator of competition and predation. Science, 211 (4484), 846–848. https://doi.org/10.1126/science.211.4484.846
- Parapar, J., Moreira, J., Gambi, M.C. & Caramelo, C. (2013) Morphology and biology of Laetmonice producta producta Grube (Polychaeta: Aphroditidae) in the Bellingshausen Sea and Antarctic Peninsula (Southern Ocean, Antarctica). Italian Journal of Zoology, 80 (2), 255–272. https://doi.org/10.1080/11250003.2012.758783
- Pernet, B. (2000) A scaleworm setal snorkel. Invertebrate Biology, 119 (2), 147–151. https://doi.org/10.1111/j.1744-7410.2000.tb00003.x
- Pettibone, M.H. (1993) Scaled Polychaetes (Polynoidae) associated with ophiuroids and other invertebrates and review of species referred to Malmgrenia McIntosh and replaced by Malmgreniella Hartman, with descriptions of new taxa. Smithsonian Contributions to Zoology, 538, 1–92. https://doi.org/10.5479/si.00810282.538
- Pettibone, M.H. (1997) Revision of the sigalionid species (Polychaeta) referred to Psammolyce Kinberg, 1856, Pelogenia Schmarda, 1861, and belonging to the subfamily Pelogeniinae Chamberlin, 1919. Smithsonian Contributions to Zoology, 581, 1–89. https://doi.org/10.5479/si.00810282.581
- Piraino, S., Bouillon, J. & Boero, F. (1992) Halocoryne epizoica (Cnidaria, Hydrozoa), a hydroid that ‘bites’. Scientia Marina, 56 (2–3), 141–147.
- Schmarda, L.K. (1861) Neue wirbellose Thiere beobachtet und gesammelt auf einer Reise um die Erde 1853 bis 1857. Haelfte 2. Turbellarien, Rotatorien und Anneliden. W. Engelmann, Leipzig, 164 pp.
- Schuchert, P. (2007) The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera Part 2. Le Revue Suisse de Zoologie, 114, 195–396. https://doi.org/10.5962/bhl.part.80395
- Taboada, S., Serra Silva, A., Neal, L., Cristobo, J., Ríos, P., Álvarez-Campos, P., Hestetun, J.T., Koutsouveli, V., Sherlock, E. & Riesgo, A. (2020) Insights into the symbiotic relationship between scale worms and carnivorous sponges (Cladorhizidae, Chondrocladia). Deep-Sea Research part I: Oceanographic Research Papers, 156, 103191. https://doi.org/10.1016/j.dsr.2019.103191
- Taboada, S., Serra Silva, A., Diéz-Vives, C., Neal, L., Cristobo, J., Ríos, P., Hestetun, J.T., Clark, B., Rossi, M.E., Junoy, J., Navarro, J. & Riesgo, A. (2021) Sleeping with the enemy: unraveling the symbiotic relationships between the scale worm Neopolynoe chondrocladiae (Annelida: polynoidae) and its carnivorous sponge hosts. Zoological Journal of the Linnean Society, 193 (1), 295–318. https://doi.org/10.1093/zoolinnean/zlaa146
- Wahl, M. (1997) Living attached: Aufwuchs, fouling, epibiosis. In: Nagabhushanam, R. & Thompson, M.F. (Eds.), Fouling organisms of the Indian Ocean: biology and control technology. Oxford & IBH Publ., New Delhi, pp. 31–83. https://doi.org/10.1201/9781003077992-2
- Wahl, M. (2008) Ecological lever and interface ecology: epibiosis modulates the interactions between host and environment. Biofouling, 24 (6), 427–438. https://doi.org/10.1080/08927010802339772
- Wehe, T. (2007) Revision of the scale worms (Polychaeta: Aphroditoidea) occurring in the seas surrounding the Arabian Peninsula. Peninsula. Part II. Sigalionidae. Fauna of Arabia, 23, 41–124.
- Widmer, C.L., Cailliet, G. & Geller, J. (2009) The life cycle of Earleria corachloeae n. sp. (Cnidaria: Hydrozoa) with epibiotic hydroids on mid-water shrimp. Marine Biology, 157, 49–58. https://doi.org/10.1007/s00227-009-1294-y
- WoRMS Editorial Board (2024) Bimeria Wright, 1859, World Register of Marine Species. Available from: http://www.marinespecies.org/aphia.php?p=taxdetails&id=1337 (accessed 21 October 2024)