Abstract
With the advent of molecular data, the discovery of cryptic species has become commonplace. New Guinea, a region of high vertebrate biodiversity and complex geological history, has been found to contain immense numbers of cryptic skink species. We present the first molecular phylogenetic analysis of Carlia Gray, 1845, and its sister genus Lygisaurus de Vis, 1884, across mainland New Guinea and the Solomon Islands. We find rainbow skinks exhibit significant genetic divergence with minimal morphological variation and our data suggest the existence of many undescribed species. Due to the morphologically cryptic nature of rainbow skinks, we demonstrate the efficacy of the COI gene as a “barcode” for difficult species determinations.
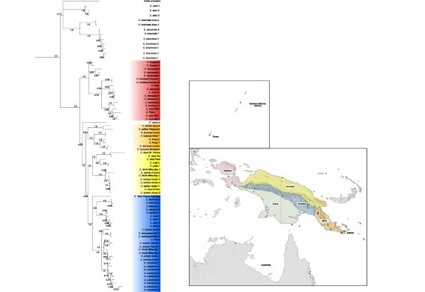
Divergence time and biogeographic analyses support four separate dispersal events from Australia to New Guinea for rainbow skinks from ~10–5 mya, with most groups arriving first in the East Papua Composite Terrane (EPCT) and dispersing from there to other terranes and islands. Exceptions to this pattern were observed in Lygisaurus, which dispersed first to the West Papuan portion of the Craton ~8.4mya, and the island clade of the Carlia fusca group, which dispersed to the Vogelkop peninsula ~4.7mya and from there to many islands.
References
- Adams, M., Raadik, T.A., Burridge, C.P. & Georges, A. (2014) Global biodiversity assessment and hyper-cryptic species complexes: more than one species of elephant in the room? Systematic Biology, 63 (4), 51–533. https://doi.org/10.1093/sysbio/syu017
- Adler, G.H., Austin, C.C. & Dudley, R. (1995) Dispersal and speciation of skinks among archipelagos in the tropical Pacific Ocean. Evolutionary Ecology, 9, 529–541. https://doi.org/10.1007/BF01237834
- Afonso Silva, A.C., Bragg, J.G., Potter, S., Fernandes, C., Coelho, M.M. & Moritz, C. (2017) Tropical specialist vs. climate generalist: diversification and demographic history of sister species of Carlia skinks from northwestern Australia. Molecular Ecology, 26 (15), 4045–4058. https://doi.org/10.1111/mec.14185
- Allison, A. & Greer, A.E. (1986) Egg shells with pustulate surface structures: basis for a new genus of New Guinea skinks (Lacertilia: Scincidae). Journal of Herpetology, 20 (1), 116–119. https://doi.org/10.2307/1564142
- Austin, C.C. (1995) Molecular and morphological evolution in South Pacific scincid lizards: morphological conservatism and phylogenetic relationships of Papuan Lipinia (Scincidae). Herpetologica, 51 (3), 291–300.
- Austin, C.C., Hayden, C.J., Bigilale, I., Dahl, C. & Anaminiato, J. (2008) Checklist and comments on the terrestrial amphibian and reptile fauna from Utai, northwestern Papua New Guinea. Herpetological Review, 39 (1), 40.
- Austin, C.C., Rittmeyer, E.N., Oliver, L.A., Andermann, J.O., Zug, G.R., Rodda, G.H. & Jackson, N.D. (2011) The bioinvasion of Guam: inferring geographic origin, pace, pattern and process of an invasive lizard (Carlia) in the Pacific using multi-locus genomic data. Biological Invasions, 13 (9), 1951–1967. https://doi.org/10.1007/s10530-011-0014-y
- Austin, C.C. & Zug, G.R. (1999) Molecular and morphological evolution in the south-central Pacific skink Emoia tongana (Reptilia: Squamata): uniformity and human-mediated dispersal. Australian Journal of Zoology, 47 (5), 425–437. https://doi.org/10.1071/ZO99019
- Baldwin, S.L., Fitzgerald, P.G. & Webb, L.E. (2012) Tectonics of the New Guinea region. Annual Review of Earth and Planetary Sciences, 40 (1), 495–520. https://doi.org/10.1146/annurev-earth-040809-152540
- Barley, A.J., White, J., Diesmos, A.C. & Brown, R.M. (2013) The challenge of species delimitation at the extremes: diversification without morphological change in Philippine sun skinks. Evolution, 67 (12), 3556–3572. https://doi.org/10.1111/evo.12219
- Bird, P. (2003) An updated digital model of plate boundaries. Geochemistry, Geophysics, Geosystems, 4 (3), 1–52. https://doi.org/10.1029/2001GC000252
- Blomberg, S.P. & Stuart-Fox, D. (2001) Ultraviolet reflectance in the small skink Carlia pectoralis. Herpetological Review, 32 (1), 16.
- Boulenger, G.A. (1898) An account of the reptiles and batrachians collected by Dr. L. Loria in British New Guinea. Annali del Museo Civico di Storia Naturale di Genova, 38 (2), 694–710.
- Bouckaert, R., Vaughan, T.G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., De Maio, N., Matschiner, N., Mendes, F.K., Müller, N.F., Ogilvie, H.A., du Plessis, L., Popinga, A., Rambaut, A., Rasmussen, D., Siveroni, I., Suchard, M.A., Wu, C., Xie, D., Zhang, C., Stadler, T. & Drummond, A.J. (2019) BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS computational biology, 15 (4), e1006650. https://doi.org/10.1371/journal.pcbi.1006650
- Bragg, J.G., Potter, S., Afonso Silva, A.C., Hoskin, C.J., Bai, B.Y. & Moritz, C. (2018) Phylogenomics of a rapid radiation: the Australian rainbow skinks. BMC evolutionary biology, 18 (15), 1–12. https://doi.org/10.1186/s12862-018-1130-4
- Brandley, M.C., Bragg, J.G., Singhal, S., Chapple, D.G., Jennings, C.K., Lemmon, A.R., Lemmon, E.M., Thompson, M.B. & Moritz, C. (2015) Evaluating the performance of anchored hybrid enrichment at the tips of the tree of life: a phylogenetic analysis of Australian Eugongylus group scincid lizards. BMC evolutionary biology, 15 (62), 1–14. https://doi.org/10.1186/s12862-015-0318-0
- Bruna, E.M., Fisher, R.N. & Case, T.J. (1996) Morphological and genetic evolution appear decoupled in Pacific skinks (Squamata: Scincidae: Emoia). Proceedings of the Royal Society of London. Series B: Biological Sciences, 263 (1371), 681–688. https://doi.org/10.1098/rspb.1996.0102
- Bryant, D. & Moulton, V. (2004) Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Molecular biology and evolution, 21 (2), 255–265. https://doi.org/10.1093/molbev/msh018
- Buden, D.W. (2009) Carlia ailanpalai (Reptilia: Scincidae): An Invasive Species of Lizard in the Federated States of Micronesia. Pacific Science, 63 (2), 243–251. https://doi.org/10.2984/049.063.0206
- Cámara-Leret, R., Frodin, D.G., Adema, F., Anderson, C., Appelhans, M.S., Argent, G., Arias Guerrero, S., Ashton, P., Baker, W.J., Barfod, A.S., Barrington, D., Borosova, R., Bramley, G.L., Briggs, M., Buerki, S., Cahen, D., Callmander, M.W., Cheek, M., Chen, C.W., Conn, B.J., Coode, M.J., Darbyshire, I., Dawson, S., Dransfield, J., Drinkell, C., Duyfjes, B., Ebihara, A., Ezedin, Z., Fu, L.F., Gideon, O., Girmansyah, D., Govaerts, R., Fortune-Hopkins, H., Hassemer, G., Hay, A., Heatubun, C.D., Hind, D.J., Hoch, P., Homot, P., Hovenkamp, P., Hughes, M., Jebb, M., Jennings, L., Jimbo, T., Kessler, M., Kiew, R., Knapp, S., Lamei, P., Lehnert, M., Lewis, G.P., Linder, H.P., Lindsay, S., Low, Y.W., Lucas, E., Mancera, J.P., Monro, A.K., Moore, A., Middleton, D.J., Nagamasu, H., Newman, M.F., Nic Lughadha, E., Melo, P.H., Ohlsen, D.J., Pannell, C.M., Parris, B., Pearce, L., Penneys, D.S., Perrie, L.R., Petoe, P., Poulsen, A.D., Prance, G.T., Quakenbush, J.P., Raes, N., Rodda, M., Rogers, Z.S., Schuiteman, A., Schwartsburd, P., Scotland, R.W., Simmons, M.P., Simpson, D.A., Stevens, P., Sundue, M., Testo, W., Trias-Blasi, A., Turner, I., Utteridge, T., Walsingham, L., Webber, B.L., Wei, R., Weiblen, G.D., Weigend, M., Weston, P., de Wilde, W., Wilkie, P., Wilmot-Dear, C.M., Wilson, H.P., Wood, J.R., Zhang, L. & van Welzen, P.C. (2020) New Guinea has the world’s richest island flora. Nature, 584 (7822), 579–583. https://doi.org/10.1038/s41586-020-2549-5
- Cerca, J., Meyer, C., Stateczny, D., Siemon, D., Wegbrod, J., Purschke, G., Dimitrov, D. & Struck, T.H. (2020) Deceleration of morphological evolution in a cryptic species complex and its link to paleontological stasis. Evolution, 74 (1), 116–131. https://doi.org/10.1111/evo.13884
- Chapple, D.G., Slavenko, A., Tingley, R., Farquhar, J.E., Camaiti, M., Roll, U. & Meiri, S. (2023) Built for success: Distribution, morphology, ecology and life history of the world’s skinks. Ecology and Evolution, 13 (12), e10791. https://doi.org/10.1002/ece3.10791
- Chapple, D.G., Chapple, S.N., Smith, S.A., Shea, G.M., Brennan, I.G. & Sadlier, R.A. (2023) Phylogenetic relationships in the Eugongylini (Squamata: Scincidae): generic limits and biogeography. Australian Journal of Zoology, 70 (6), 165–203. https://doi.org/10.1071/ZO23007
- Cogger, H.G. (2014) Reptiles and Amphibians of Australia. CSIRO Publishing, Collingwood, 1033 pp. https://doi.org/10.1071/9780643109773
- Couper, P.J., Wilmer, J.W., Roberts, L., Amey, A.P. & Zug, G.R. (2005) Skinks currently assigned to Carlia aerata (Scincidae: Lygosominae) of north-eastern Queensland: a preliminary study of cryptic diversity and two new species. Australian Journal of Zoology, 53 (1), 35–49. https://doi.org/10.1071/ZO04010
- Davies, H.L. (2012) The geology of New Guinea-the cordilleran margin of the Australian continent. Episodes Journal of International Geoscience, 35 (1), 87–102. https://doi.org/10.18814/epiiugs/2012/v35i1/008
- Davies, H.L., Perembo, R.C., Winn, R.D. & KenGemar, P. (1997) Terranes of the New Guinea orogen. In: Hancock, G. (Ed.), Proceedings of the Geology Exploration and Mining Conference, Madang. Australasian Institute of Mining and Metallurgy, Melbourne, pp. 61-66.
- Davies, H.L., Winn, R.D. & KenGemar, P. (1996) Evolution of the Papuan Basin-a view from the orogen. Papua New Guinea (PNG) Petroleum Convention Proceedings. In: Buchanan, P. (Ed.), Petroleum exploration, development and production in Papua New Guinea. PNG Chamber of Mines and Petroleum, Port Moresby, pp. 53–62.
- De Vis, C. (1884) New Queensland lizards. Proceedings of the Royal Society of Queensland, 1 (2), 77–78. https://doi.org/10.5962/p.351005
- Dolman, G. & Hugall, A.F. (2008) Combined mitochondrial and nuclear data enhance resolution of a rapid radiation of Australian rainbow skinks (Scincidae: Carlia). Molecular Phylogenetics and Evolution, 49 (3), 782–794. https://doi.org/10.1016/j.ympev.2008.09.021
- Dolman, G. & Stuart-Fox, D. (2010) Processes driving male breeding colour and ecomorphological diversification in rainbow skinks: a phylogenetic comparative test. Evolutionary Ecology, 24, 97–113. https://doi.org/10.1007/s10682-009-9293-5
- Donnellan, S., Couper, P., Saint, K. & Wheaton, L. (2009) Systematics of the Carlia fusca complex (Reptilia: Scincidae) from northern Australia. Zootaxa, 2227 (1), 1–31. https://doi.org/10.11646/zootaxa.2227.1.1
- Dow, D.B. (1972) Geology of the South Sepik Region, New Guinea. Bulletin/Dept. of National Development, Bureau of Mineral Resources, Geology and Geophysics; 133. Australian Government Publishing Service, Canberra, 88 pp.
- Duméril, A.M.C. & Bibron, G. (1841) Erpétologie Générale ou Histoire Naturelle Compléte des Reptiles. Vol. 8. Roret, Paris, 792 pp.
- Drummond, A.J., Suchard, M.A., Xie, D. & Rambaut, A. (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular biology and evolution, 29 (8), 1969–1973. https://doi.org/10.1093/molbev/mss075
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research, 32 (5), 1792–1797. https://doi.org/10.1093/nar/gkh340
- Eme, D., Zagmajster, M., Delić, T., Fišer, C., Flot, J.F., Konecny‐Dupré, L., Pálsson, S., Stoch, F., Zakšek, V., Douady, C.J. & Malard, F. (2018) Do cryptic species matter in macroecology? Sequencing European groundwater crustaceans yields smaller ranges but does not challenge biodiversity determinants. Ecography, 41 (2), 424–436. https://doi.org/10.1111/ecog.02683
- Escoriza, D. (2023) Success factors of great oceanic dispersers: Case of Squamata in the Pacific Ocean. Journal of Zoology, 319 (3), 221–230. https://doi.org/10.1111/jzo.13042
- Fleishman, L.J., Loew, E.R. & Leal, M. (1993) Ultraviolet vision in lizards. Nature, 365 (6445), 397. https://doi.org/10.1038/365397a0
- Fišer, C., Robinson, C.T. & Malard, F. (2018) Cryptic species as a window into the paradigm shift of the species concept. Molecular Ecology, 27 (3), 613–635. https://doi.org/10.1111/mec.14486
- Fitzinger, L. (1843) Systema Reptilium. Fasciculus primus, Amblyglossae. Braumüller et Seidel, Vienna, 106 pp. https://doi.org/10.5962/bhl.title.4694
- Funk, W.C., Caminer, M. & Ron, S.R. (2012) High levels of cryptic species diversity uncovered in Amazonian frogs. Proceedings of the Royal Society B: Biological Sciences, 279 (1734), 1806–1814. https://doi.org/10.1098/rspb.2011.1653
- Georges, A., Zhang, X., Unmack, P., Reid, B.N., Le, M. & McCord, W.P. (2014) Contemporary genetic structure of an endemic freshwater turtle reflects Miocene orogenesis of New Guinea. Biological Journal of the Linnean Society, 111 (1), 192–208. https://doi.org/10.1111/bij.12176
- Goldberg, S.R. & Kraus, F. (2012) Reproduction in the invasive lizard, Carlia ailanpalai (Squamata: Scincidae) from Oceania. Russian Journal of Herpetology, 19 (3), 199–202.
- Gray, J.E. (1845) Cataloge of the specimens of lizards in the collection of the British Museum. Trustees of the British Museum, London, 289 pp. https://doi.org/10.5962/bhl.title.5499
- Greer, A.E. (1974) The genetic relationships of the scincid lizard genus Leiolopisma and its relatives. Australian Journal of Zoology Supplementary Series, 22 (31), 1–67. https://doi.org/10.1071/AJZS031
- Günther, A.C.L.G. (1858) On the systematic arrangement of the tailless batrachians and the structure of Rhinophrynus dorsalis. Proceedings of the Zoological Society of London, 1858, 339–352. https://doi.org/10.1111/j.1469-7998.1858.tb06387.x
- Hall, R. (1998) The plate tectonics of Cenozoic SE Asia and the distribution of land and sea. In: Hall, R. & Holloway, J. (Eds.), Biogeography and Geological Evolution of SE Asia. Backhuys, Leiden, pp. 99–131.
- Hall, R. (2002) Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. Journal of Asian earth sciences, 20 (4), 353–431. https://doi.org/10.1016/S1367-9120(01)00069-4
- Hall, R. & Spakman, W. (2003) Mantle structure and tectonic evolution of the region north and east of Australia. Special Papers-Geological Society of America, 372, 361–382. https://doi.org/10.1130/0-8137-2372-8.361
- Hill, E.C., Gao, D.F., Polhemus, D.A., Fraser, C.J., Iova, B., Allison, A. & Butler, M.A. (2023) Testing geology with biology: plate tectonics and the diversification of microhylid frogs in the Papuan region. Integrative Organismal Biology, 5 (1), obad028 https://doi.org/10.1093/iob/obad028
- Hoskin, C.J. & Couper, P.J. (2015) A new skink (Scincidae: Liburnascincus) from rocky habitat on Cape York, northeast Australia. Zootaxa, 3994 (2), 222–234. https://doi.org/10.11646/zootaxa.3994.2.3
- Huson, D.H. & Bryant, D. (2006) Application of phylogenetic networks in evolutionary studies. Molecular biology and evolution, 23 (2), 254–267. https://doi.org/10.1093/molbev/msj030
- Ingram, G. & Covacevich, J. (1988) Revision of the genus Lygisaurus de Vis (Scincidae: Reptilia) in Australia. Memoirs of the Queensland Museum, 25 (2), 335–354.
- Ingram, G. & Covacevich, J. (1989) Revision of the genus Carlia (Reptilia, Scincidae) in Australia with comments on Carlia bicarinata of New Guinea. Memoirs of the Queensland Museum, 27 (2), 443–490.
- Jackson, D.A. (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology, 74 (8), 2204–2214. https://doi.org/10.2307/1939574
- Johnson, T. & Molnar, P. (1972) Focal mechanisms and plate tectonics of the southwest Pacific. Journal of Geophysical Research, 77 (26), 5000–5032. https://doi.org/10.1029/JB077i026p05000
- Knowlton, N. (1993) Sibling species in the sea. Annual review of ecology and systematics, 24, 189-216. https://doi.org/10.1146/annurev.es.24.110193.001201
- Kopstein, P.F. (1926) Reptilien von den Molukken und den benachbarten Inseln. Zoologische Mededelingen, 9 (5), 71–112.
- Kornilios, P., Kumlutaş, Y., Lymberakis, P. & Ilgaz, Ç. (2018) Cryptic diversity and molecular systematics of the Aegean Ophiomorus skinks (Reptilia: Squamata), with the description of a new species. Journal of Zoological Systematics and Evolutionary Research, 56 (3), 364–381. https://doi.org/10.1111/jzs.12205
- Korshunova, T., Martynov, A., Bakken, T. & Picton, B. (2017) External diversity is restrained by internal conservatism: new nudibranch mollusc contributes to the cryptic species problem. Zoologica Scripta, 46 (6), 683–692. https://doi.org/10.1111/zsc.12253
- Kraus, F. (2007) Taxonomic partitioning within Papuan members of the Carlia novaeguineae complex (Squamata: Scincidae). Journal of Herpetology, 41 (3), 410–423. https://doi.org/10.1670/0022-1511(2007)41[410:TPWPMO]2.0.CO;2
- Kurita, K. & Hikida, T. (2014) Divergence and long-distance overseas dispersals of island populations of the Ryukyu five-lined skink, Plestiodon marginatus (Scincidae: Squamata), in the Ryukyu Archipelago, Japan, as revealed by mitochondrial DNA phylogeography. Zoological science, 31 (4), 187–194. https://doi.org/10.2108/zs130179
- Lavoué, S., Miya, M., Arnegard, M.E., McIntyre, P.B., Mamonekene, V. & Nishida, M. (2011) Remarkable morphological stasis in an extant vertebrate despite tens of millions of years of divergence. Proceedings of the Royal Society B: Biological Sciences, 278 (1708), 1003–1008. https://doi.org/10.1098/rspb.2010.1639
- Letunic, I. & Bork, P. (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic acids research, 49 (W1), W293–W296. https://doi.org/10.1093/nar/gkab301
- Li, X. & Wiens, J.J. (2023) Estimating global biodiversity: the role of cryptic insect species. Systematic Biology, 72 (2), 391–403. https://doi.org/10.1093/sysbio/syac069
- Lindley, D. (1988) Early Cainozoic stratigraphy and structure of the Gazelle Peninsula, east New Britain: an example of extensional tectonics in the New Britain arc-trench complex. Journal of the Geological Society of Australia, 35 (2), 231–244. https://doi.org/10.1080/14400958808527943
- Linkem, C.W., Hesed, K.M., Diesmos, A.C. & Brown, R.M. (2010) Species boundaries and cryptic lineage diversity in a Philippine forest skink complex (Reptilia; Squamata; Scincidae: Lygosominae). Molecular Phylogenetics and Evolution, 56 (2), 572–585. https://doi.org/10.1016/j.ympev.2010.03.043
- Loew, E., Govardovskii, V., Röhlich, P. & Szel, A. (1996) Microspectrophotometric and immunocytochemical identification of ultraviolet photoreceptors in geckos. Visual Neuroscience, 13 (2), 247–256. https://doi.org/10.1017/S0952523800007483
- Loew, E.R. (1994) A third, ultraviolet-sensitive, visual pigment in the Tokay gecko (Gekko gekko). Vision research, 34 (11), 1427–1431. https://doi.org/10.1016/0042-6989(94)90143-0
- Lukić, D., Waterkeyn, A., Rabet, N., Mioduchowska, M., Geudens, B., Vanschoenwinkel, B., Brendonck L. & Pinceel, T. (2019) High genetic variation and phylogeographic relations among Palearctic fairy shrimp populations reflect persistence in multiple southern refugia during Pleistocene ice ages and postglacial colonisation. Freshwater Biology, 64 (11), 1896–1907. https://doi.org/10.1111/fwb.13380
- Macleay, W. (1877) The lizards of the “Chevert” Expedition. Proceedings of the Linnean Society of New South Wales, 2 (1), 60–69. https://doi.org/10.5962/bhl.part.12420
- Martin, M., Le Galliard, J.F., Meylan, S. & Loew, E.R. (2015) The importance of ultraviolet and near-infrared sensitivity for visual discrimination in two species of lacertid lizards. Journal of Experimental Biology, 218 (3), 458–465. https://doi.org/10.1242/jeb.115923
- Matzke, N.J. (2013) Probabilistic historical biogeography: New models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Frontiers in Biogeography, 5, 242–248. https://doi.org/10.1093/sysbio/syu056
- Matzke, N. J. (2014) Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Systematic biology, 63 (6), 951–970. https://doi.org/10.1093/sysbio/syu056
- Meyer, A. (1874) Platemys novaeguineae sp. nov. Dr. WH Peters legte vor: Eine mittheilung von Hrn. Adolf Bernhard Meyer uber die von ihm auf Neu-Guinea under den Inseln Jobi, Mysore und Mafoor im Jahre 1873 gesammelten Amphibien. Monatsberichte der Koniglich Preussischen Akademie der Wissenschaften zu Berlin, 39, 128–140.
- Mittermeier, R.A., Mittermeier, C.G., Brooks, T.M., Pilgrim, J.D., Konstant, W.R., Da Fonseca, G.A. & Kormos, C. (2003) Wilderness and biodiversity conservation. Proceedings of the National Academy of Sciences, 100 (18), 10309–10313. https://doi.org/10.1073/pnas.1732458100
- Monro, A.K. & Mayo, S.J. (2022) Cryptic species: Morphological stasis, circumscription, and hidden diversity. Cambridge University Press, Cambridge, 350 pp. https://doi.org/10.1017/9781009070553
- Nguyen, L.T., Schmidt, H.A., Von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular biology and evolution, 32 (1), 268–274. https://doi.org/10.1093/molbev/msu300
- Oliver, P.M., Adams, M., Lee, M.S., Hutchinson, M.N. & Doughty, P. (2009) Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota). Proceedings of the Royal Society B: Biological Sciences, 276 (1664), 2001–2007. https://doi.org/10.1098/rspb.2008.1881
- Oliver, P.M., Kraus, F., Austin, C.C., Tedeschi, L.G., O’Brien, A.R. & Maddock, S.T. (2024) Lineage diversity in a Melanesian lizard radiation (Gekkonidae: Nactus) further highlights exceptional diversity and endemism in eastern Papua New Guinea. Organisms Diversity & Evolution, 2024, 1–16. https://doi.org/10.1007/s13127-024-00655-w
- Paradis, E. & Schliep, K. (2019) “ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R.” Bioinformatics, 35 (3), 526–528. https://doi.org/10.1093/bioinformatics/bty633
- Pérez-Ponce de León, G. & Poulin, R. (2016) Taxonomic distribution of cryptic diversity among metazoans: not so homogeneous after all. Biology letters, 12 (8), 20160371. https://doi.org/10.1098/rsbl.2016.0371
- Peters, W. (1864) Die Eidechsenfamilie der Scincoiden, insbesonder euber die Schneider’schen. Wiegmann’schen und neue Arten des zoologishen Museums, 1864, 44-58.
- Peters, W., Beccari, O., Albertis, L., Bruijn, A. & Doria, G. (1878) Catalogo dei rettili e dei batraci raccolti da O. Beccari, LM D’Albertis e AA Bruijn nella Sotto-Regione Austro-Malese. Annali del Museo Civico de Storia Naturale di Genova, Series 1, 13, 323–450.
- Pfenninger, M. & Schwenk, K. (2007) Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC evolutionary biology, 7 (1), 121. https://doi.org/10.1186/1471-2148-7-121
- Pigram, C. & Davies, H.L. (1987) Terranes and the accretion history of the Papua New Guinea Orogen. Australian Bureau of Mineral Resources, Geology and Geophysics Bulletin, 10, 193–211.
- Polhemus, D.A. & Polhemus, J.T. (1998) Assembling New Guinea: 40 million years of island arc accretion as indicated by the distributions of aquatic Heteroptera (Insecta). In: Hall, R. & Holloway, J. (Eds.), Biogeography and Geological Evolution of SE Asia. Backhuys, Leiden, pp. 327–340.
- Potter, S., Bragg, J.G., Peter, B.M., Bi, K. & Moritz, C. (2016) Phylogenomics at the tips: inferring lineages and their demographic history in a tropical lizard, Carlia amax. Molecular Ecology, 25 (6), 1367–1380. https://doi.org/10.1111/mec.13546
- Prates, I., Doughty, P. & Rabosky, D.L. (2023a) Subspecies at crossroads: the evolutionary significance of genomic and phenotypic variation in a wide-ranging Australian lizard (Ctenotus pantherinus). Zoological Journal of the Linnean Society, 197 (3), 768–786. https://doi.org/10.1093/zoolinnean/zlac076
- Prates, I., Hutchinson, M.N., Singhal, S., Moritz, C. & Rabosky, D.L. (2023b) Notes from the taxonomic disaster zone: Evolutionary drivers of intractable species boundaries in an Australian lizard clade (Scincidae: Ctenotus). Molecular Ecology, 33 (20), e17074. https://doi.org/10.1111/mec.17074
- R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available from: http://www.R-project.org/ (accessed 9 May 2024)
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic biology, 67 (5), 901–904. https://doi.org/10.1093/sysbio/syy032
- Ratnasingham, S. & Hebert, P.D. (2007) BOLD: The Barcode of Life Data System (http://www. barcodinglife. org). Molecular ecology notes, 7 (3), 355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
- Rausch, T., Fritz, M.H., Untergasser, A. & Benes, V. (2020) Tracy: basecalling, alignment, assembly and deconvolution of sanger chromatogram trace files. BMC Genomics, 21, 230. https://doi.org/10.1186/s12864-020-6635-8
- Ree, R.H. & Sanmartín, I. (2018) Conceptual and statistical problems with the DEC+ J model of founder‐event speciation and its comparison with DEC via model selection. Journal of Biogeography, 45 (4), 741–749. https://doi.org/10.1111/jbi.13173
- Reidenbach, K.R., Neafsey, D.E., Costantini, C., Sagnon, N.F., Simard, F., Ragland, G.J., Egan, S.P., Feder, J.L., Muskavitch, M.A. & Besansky, N.J. (2012) Patterns of genomic differentiation between ecologically differentiated M and S forms of Anopheles gambiae in West and Central Africa. Genome biology and evolution, 4 (12), 1202–1212. https://doi.org/10.1093/gbe/evs095
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61 (3), 539–542. https://doi.org/10.1093/sysbio/sys029
- Schenk, J.J. (2016) Consequences of secondary calibrations on divergence time estimates. PLoS ONE, 11 (1), e0148228. https://doi.org/10.1371/journal.pone.0148228
- Skinner, A., Hutchinson, M.N. & Lee, M.S. (2013) Phylogeny and divergence times of Australian Sphenomorphus group skinks (Scincidae, Squamata). Molecular Phylogenetics and Evolution, 69 (3), 906–918. https://doi.org/10.1016/j.ympev.2013.06.014
- Slavenko, A., Allison, A., Austin, C.C., Bauer, A.M., Brown, R.M., Fisher, R.N., Ineich, I., Iova, B., Karin, B.R., Kraus, F., Mecke, S., Meiri, S., Morrison, C., Oliver, P.M., O’Shea, M., Richmond, J.Q., Shea, G.M., Tallowin, O.J. & Chapple, D.G. (2023) Skinks of Oceania, New Guinea, and Eastern Wallacea: an underexplored biodiversity hotspot. Pacific Conservation Biology, 29 (6), 526–543. https://doi.org/10.1071/PC22034
- Slavenko, A., Allison, A. & Meiri, S. (2021) Elevation is a stronger predictor of morphological trait divergence than competition in a radiation of tropical lizards. Journal of Animal Ecology, 90 (4), 917–930. https://doi.org/10.1111/1365-2656.13420
- Slavenko, A., Tamar, K., Tallowin, O.J., Allison, A., Kraus, F., Carranza, S. & Meiri, S. (2020) Cryptic diversity and non-adaptive radiation of montane New Guinea skinks (Papuascincus; Scincidae). Molecular Phylogenetics and Evolution, 146, 106749. https://doi.org/10.1016/j.ympev.2020.106749
- Slavenko, A., Tamar, K., Tallowin, O.J., Kraus, F., Allison, A., Carranza, S. & Meiri, S. (2022) Revision of the montane New Guinean skink genus Lobulia (Squamata: Scincidae), with the description of four new genera and nine new species. Zoological Journal of the Linnean Society, 195 (1), 220–278. https://doi.org/10.1093/zoolinnean/zlab052
- Stattersfield, A. (1998) Identifying threatened species in the” south” using new criteria. Pacific Conservation Biology, 4 (1), 33–38. https://doi.org/10.1071/PC980033
- Storr, G. (1974) The genus Carlia (Lacertilia, Scincidae) in Western Australia and the Northern Territory. Records of the Western Australian Museum, 3 (2), 151–165.
- Struck, T.H., Feder, J.L., Bendiksby, M., Birkeland, S., Cerca, J., Gusarov, V.I., Kistenich, S., Larsson, K.H., Liow, L.H., Nowak, M.D., Stedje, B., Bachmann, L. & Dimitrov, D. (2018) Finding evolutionary processes hidden in cryptic species. Trends in Ecology & Evolution, 33 (3), 153–163. https://doi.org/10.1016/j.tree.2017.11.007
- Stuart-Fox, D.M., Hugall, A.F. & Moritz, C. (2002) A molecular phylogeny of rainbow skinks (Scincidae: Carlia): taxonomic and biogeographic implications. Australian Journal of Zoology, 50 (1), 39–51. https://doi.org/10.1071/ZO01051
- Tallowin, O.J., Tamar, K., Meiri, S., Allison, A., Kraus, F., Richards, S.J. & Oliver, P.M. (2018) Early insularity and subsequent mountain uplift were complementary drivers of diversification in a Melanesian lizard radiation (Gekkonidae: Cyrtodactylus). Molecular Phylogenetics and Evolution, 125, 29–39. https://doi.org/10.1016/j.ympev.2018.03.020
- Tamura, K., Stecher, G. & Kumar, S. (2021) MEGA11: molecular evolutionary genetics analysis version 11. Molecular biology and evolution, 38 (7), 3022–3027. https://doi.org/10.1093/molbev/msab120
- Toussaint, E.F., Hall, R., Monaghan, M.T., Sagata, K., Ibalim, S., Shaverdo, H.V., Vogler, A.P., Pons, J. & Balke, M. (2014) The towering orogeny of New Guinea as a trigger for arthropod megadiversity. Nature communications, 5 (1), 4001. https://doi.org/10.1038/ncomms5001
- Vacher, J.P., Chave, J., Ficetola, F.G., Sommeria-Klein, G., Tao, S., Thébaud, C., Blanc, M., Camacho, A., Cassimiro, J., Colston, T.J., Dewynter, M., Ernst, R., Gaucher, P., Gomes, J.O., Jairam, R., Kok, P.J., Lima, J.D., Martinez, Q., Marty, C., Noonan, B.P., Sales Nunes, P.M., Ouboter, P., Recoder, R., Rodrigues, M.T., Snyder, A., Marques-Souza, S. & Fouquet, A. (2020) Large‐scale DNA‐based survey of frogs in Amazonia suggests a vast underestimation of species richness and endemism. Journal of Biogeography, 47 (8), 1781–1791. https://doi.org/10.1111/jbi.13847
- van Ufford, A.Q. & Cloos, M. (2005) Cenozoic tectonics of new Guinea. AAPG Bulletin, 89 (1), 119–140. https://doi.org/10.1306/08300403073
- Voje, K.L., Starrfelt, J. & Liow, L.H. (2018) Model adequacy and microevolutionary explanations for stasis in the fossil record. The American Naturalist, 191 (4), 509–523. https://doi.org/10.1086/696265
- Wells, R.W. & Wellington, C.R. (1984) A synopsis of the class Reptilia in Australia. Australian Journal of Herpetology, 1, 73–129.
- Whiting, A.S., Sites Jr, J.W., Pellegrino, K.C. & Rodrigues, M.T. (2006) Comparing alignment methods for inferring the history of the new world lizard genus Mabuya (Squamata: Scincidae). Molecular Phylogenetics and Evolution, 38 (3), 719–730. https://doi.org/10.1016/j.ympev.2005.11.011
- Zug, G.R. (2004) Systematics of the Carlia “fusca” lizards (Squamata:Scincidae) of New Guinea and nearby islands. Bishop Museum Bulletin in Zoology, 5, 1–84
- Zug, G.R. (2010) An outlying Carlia population from Java and comments on species groups within the genus Carlia (Reptilia: Squamata: Scincidae). Proceedings of the California Academy of Sciences, 61 (8), 389–408
- Zug, G.R. & Allison, A. (2006) New Carlia fusca complex lizards (Reptilia: Squamata: Scincidae) from New Guinea, Papua-Indonesia. Zootaxa, 1237 (1), 27–44 https://doi.org/10.11646/zootaxa.1237.1.3