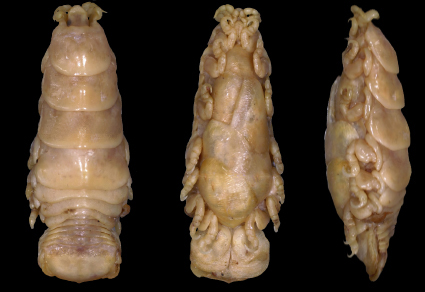
Abstract
Lobothorax bharat sp. nov., is described based on specimens collected in 2023 from Trichiurus lepturus Linnaeus, 1758, at the Bahabalpur and Gopalpur fish landing centres of Odisha coast, India. Lobothorax bharat sp. nov. can be identified by the sub-truncate rostrum; the anterolateral processes of pereonite 1 which are 0.45 times the cephalon length, do not extend beyond the anterior margin of cephalon; a distinct depression in the middle of pereonite 1; article 3 of maxilliped palp with three recurved robust setae; the pereopod 2 is larger than the first and the pereopod 3 being the smallest; the third pereopod is about 0.94 times that of the second and 0.97 times that of the first pereopod. Lobothorax bharat sp. nov. is further differentiated from the congeneric species L. typus in the nucleotide composition of the mitochondrial cytochrome c oxidase I (mtCOI) gene by 2.5–2.6% Kimura-2-Parameter (K2P) distance (having a base pair difference of 16–17) and belongs to a separate cluster in the Maximum Likelihood tree analysis obtained through the best-fit model.
References
- Anandkumar, A., Rameshkumar, G., Ravichandran, S., Priya, E.R., Nagarajan, R. & Leng, A.G.K. (2015) Occurrence of cymothoid isopod from Miri, East Malaysian marine fishes. Journal of Parasitic Diseases, 39 (2), 206–210. https://doi.org/10.1007/s12639-013-0320-7
- Aneesh, P.T., Bruce, N.L., Kumar, A.B., Bincy, M.R. & Sreenath, T.M. (2021) A taxonomic review of the buccal-attaching fish parasite genus Lobothorax Bleeker, 1857 (Crustacea: Isopoda: Cymothoidae) with description of a new species from southwestern India. Zoological Studies, 60, 1–13. https://doi.org/10.6620/ZS.2021.60-13
- Aneesh, P.T., Helna, A.K., Raj, S. & Kumar, A.B. (2023) Description of Elthusa aquabio sp. n. (Crustacea: Isopoda: Cymothoidae), a branchial fish parasitic isopod from Indian waters. Journal of Natural History, 57, 1193–1205. https://doi.org/10.1080/00222933.2023.2242099
- Baillie, C., Welicky, R.L., Hadfield, K.A., Smit, N.J., Mariani, S. & Beck, R.M.D. (2019) Hooked on you: shape of attachment structures in cymothoid isopods reflects parasitic strategy. BMC Evolutionary Biology, 19, 207, 1–11. https://doi.org/10.1186/s12862-019-1533-x
- Barnard, K.H. (1936) Isopods collected by the R.I.M.S. “Investigator”. Records of Indian Museum, Calcutta, 38, 147–191. https://doi.org/10.26515/rzsi/v38/i2/1936/162336
- Bleeker, P. (1857) Recherches sur les Crustacés de l’Inde archipélagique. II. Sur les Isopodes Cymothoadiens de l’archipel Indien. Natuurkundige Vereeniging in Nederlandsch-Indië, Batavia, Verhandelingen, 2, 20–40. https://doi.org/10.5962/bhl.title.9908
- Bouguerche, C., Tazerouti, F., Gey, D. & Justine, J.L. (2021) Triple barcoding for a hyperparasite, its parasitic host, and the host itself: a study of Cyclocotyla bellones (Monogenea) on Ceratothoa parallela (Isopoda) on Boops boops (Teleostei). Parasite, 28, 49. https://doi.org/10.1051/parasite/2021044
- Boyko, C.B., Bruce, N.L., Hadfield, K.A., Merrin, K.L., Ota, Y., Poore, G.C.B. & Taiti, S. (Eds.) (2024) World Marine, Freshwater and Terrestrial Isopod Crustaceans database. Lobothorax Bleeker, 1857. Accessed through: World Register of Marine Species. Available from: https://www.marinespecies.org/aphia.php?p=taxdetails&id=118409 (accessed 2 June 2024)
- Brünnich, T. (1783) Spicilegia Zoologica e Museis Naturae Curiosorum in itineribus apud exteros reportata. fol. 1765–99. World Register of Marine Species. Available from: https://www.gbif.org/fr/species/155567194/verbatim (accessed 2 June 2024)
- Bush, A.O., Lafferty, K.D., Lotz, J.M. & Shostak, A.W. (1997) Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. The Journal of Parasitology, 83 (4), 575–583. https://doi.org/10.2307/3284227
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
- Froese, R. & Pauly, D. (2024) FishBase. World Wide Web electronic publication. February 2024. Available from: https://fishbase.de/ (accessed 2 June 2024)
- Fujita, H. (2023) Morphological and Molecular Study of the Fish Parasitic Crustaceans Cymothoa indica and Mothocya collettei (Isopoda: Cymothoidae), with New Distribution Records. Diversity, 15 (9), 969. https://doi.org/10.3390/d15090969
- Fujita, H., Kawai, K., Taniguchi, R., Tomano, S., Sanchez, G., Kuramochi, T. & Umino, T. (2020) Infestation of the parasitic isopod Mothocya parvostis on juveniles of the black sea bream Acanthopagrus schlegelii as an optional intermediate host in Hiroshima Bay. Zoological Science, 37 (6), 544–553. https://doi.org/10.2108/zs190147
- Hall, T.A. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Hata, H., Sogabe, A., Tada, S., Nishimoto, R., Nakano, R., Kohya, N., Takeshima, H. & Kawanishi, R. (2017) Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Marine Biology, 164, 1–15. https://doi.org/10.1007/s00227-017-3138-5
- Hebert, P.D., Ratnasingham, S. & De Waard, J.R. (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270 (Supplement 1), S96–S99. https://doi.org/10.1098/rsbl.2003.0025
- Hebert, P.D.N., Stoeckle, M.Y., Zemlak, T.S. & Francis, C.M. (2004) Identification of Birds through DNA Barcodes. PLoS Biology, 2 (10), e312. https://doi.org/10.1371/journal.pbio.0020312
- Helna, A.K., Aneesh, P.T., Kumar, A.B. & Ohtsuka, S. (2023) Morphological description and molecular characterisation of Glyptothoa gen. nov., a fish parasitic deep-sea cymothoid (Crustacea: Isopoda) from the Indian Ocean, with four species, including one new species. Zoological Studies, 60 (51), 1–31.
- Kimura, M. (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. https://doi.org/10.1007/BF01731581
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Kusbiyanto, K., Bhagawati, D. & Nuryanto, A. (2020) DNA barcoding of Crustacean larvae in the eastern areas of Segara Anakan Cilacap, Central Java Indonesia. Biodiversitas Journal of Biological Diversity, 21 (10). [published online] https://doi.org/10.13057/biodiv/d211054
- Martin, M.B. (2015) Taxonomy and phylogeny of the buccal-attaching Cymothoidae (Crustacea: Isopoda) of Australia. Thesis, University of Tasmania, Hobart. [unknown pagination] https://doi.org/10.25959/23239826.v1
- Martin, M.B., Yusoff, M.A.M., Hashim, N.S., Do, T.D., Amin, N.M., Shaharom-Harrison, F., Sung, Y.Y., Min, W., Yantao, L., Asaduzzaman, Md. & Wong, L.L. (2024) Morphological and molecular characterisation of Cymothoa eremita (Brunnich, 1783) (Isopoda: Cymothoidae) from the South China Sea. Regional Studies in Marine Science, 71, 103372. https://doi.org/10.1016/j.rsma.2024.103372
- Mohapatra, S.K., Acharya, S., Sura, S., Mohanty, S.R., Behera, R.K., Seth, J.K., Tripathy, B. & Mohapatra, A. (2022) First report of parasitic isopod Nerocila orbignyi (Cymothoidae) from India with its molecular characterization. Journal of Parasitic Diseases, 46 (2), 483–490. https://doi.org/10.1007/s12639-022-01469-3
- Nakamura, I. & Parin, N.V. (1993) FAO species catalogue. Vol. 15. Snake mackerels and cutlassfishes of the world (Families Gempylidae and Trichiuridae). An annotated and illustrated catalogue of the snake mackerels, snoeks, escolars, gemfishes, sackfishes, domine, oilfish, cutlassfishes, scabbardfishes, hairtails, and frostfishes known to date. FAO Fisheries Synopis, 125 (15), 1–136.
- Puillandre, N., Brouillet, S. & Achaz, G. (2021) ASAP: assemble species by automatic partitioning. Molecular Ecology Resources, 21, 609–620. https://doi.org/10.1111/1755-0998.13281
- Radulovici, A.E., Sainte-Marie, B. & Dufresne, F. (2009) DNA barcoding of marine crustaceans from the Estuary and Gulf of St Lawrence: a regional‐scale approach. Molecular Ecology Resources, 9, 181–187. https://doi.org/10.1111/j.1755-0998.2009.02643.x
- Rameshkumar, G., Ravichandran, S., Sivasubramanian, K. & Trilles, J.P. (2013) New occurrence of parasitic isopods from Indian fishes. Journal of Parasitic Diseases, 37, 42–46. https://doi.org/10.1007/s12639-012-0128-x.
- Ravichandran, S., Vigneshwaran, P. & Rameshkumar, G. (2019) A taxonomic review of the fish parasitic isopod family Cymothoidae Leach, 1818 (Crustacea: Isopoda: Cymothooidea) of India. Zootaxa, 4622 (1), 1–99. https://doi.org/10.11646/zootaxa.4622.1.1
- Richardson, H. (1910) Marine isopods collected in the Philippines by the U.S. Fisheries steamer Albatross in 1907-08. Bureau of Fisheries Document, 736, 1–44. https://doi.org/10.5962/bhl.title.82673
- Schioedte, J.C. & Meinert, F. (1883) Symbolae ad monographium Cymothoarum crustaeorum isopodum familiae III. Saophridae. IV. Cerathoinae. Naturhistorisk Tidsskrift, 13, 281–378.
- Seth, J.K. & Barik, T.K. (2021) DNA Barcoding of the Family: Leiognathidae in the Water of Bay of Bengal, Odisha Coast, India Based on 16s rRNA and COI Gene Sequences. Thalassas, 37, 831–840. https://doi.org/10.1007/s41208-021-00324-1
- Seth, J.K., Mohanty, S.R., Behera, A.K., Murmu, L.K. & Mohapatra, A. (2021) First record of Lobothorax typus from Odisha coast, India, with molecular characterization and observation of its biphasic moulting. Journal of Parasitic Diseases, 45 (4), 1077–1083. https://doi.org/10.1007/s12639-021-01405-x
- Seth, J.K., Roy, S., Sura, S., Puvala, D., Mishra, S.S. & Mohapatra, A. (2023) Description of a new species of the genus Awaous Valenciennes, 1837 (Gobiiformes: Oxudercidae) from the middle stretch of the Mahanadi River, Odisha, India, with comments on the Awaous species from India. Journal of Fish Biology, 104 (3), 548–563. https://doi.org/10.1111/jfb.15598
- Stahlhut, J.K., Fernández-Triana, J., Adamowicz, S.J., Buck, M., Goulet, H., Hebert, P.D., Huber, J.T., Merilo, M.T., Sheffield, C.S., Woodcock, T. & Smith, M.A. (2013) DNA barcoding reveals diversity of Hymenoptera and the dominance of parasitoids in a sub-arctic environment. BMC ecology, 13, 1–13. https://doi.org/10.1186/1472-6785-13-2
- Tamura, K. & Nei, M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10 (3), 512–26. https://doi.org/10.1093/oxfordjournals.molbev.a040023
- Yu, H.Y. & Bruce, N.L. (2006) Redescription of Lobothorax typus bleeker, 1857 (Isopoda, Cymothoidae): the first record of the species and genus from Chinese waters. Crustaceana, 79, 641–648. https://doi.org/10.1163/156854006778026816
- Zhang, H. & Bu, W. (2022) Exploring large-scale patterns of genetic variation in the COI gene among Insecta: Implications for DNA barcoding and threshold-based species delimitation studies. Insects, 13 (5), 425. https://doi.org/10.3390/insects13050425