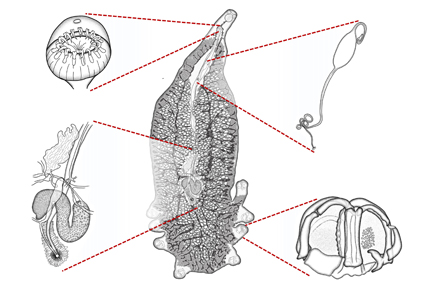
Abstract
In the last third of the 20th century, descriptions of microscopic organisms, particularly parasites, became gradually more detailed, supplemented with new morphological features. Improvements in light and electron microscopy have facilitated the description of previously unnoticed aspects of external and internal anatomy. Higher resolution allows descriptions to become increasingly detailed, together with photographs and videos providing additional information derived from museum specimens. At the same time, as technology improved, researchers began using “software” instead of traditional ink to create drawings of new species. This study introduces Yamagu-Tips, a new method for developing scientific drawings of microorganisms that can be created using Adobe Illustrator and Adobe Photoshop (Adobe Creative Cloud). This method allows automated drawing of contours, volumes, and transparencies in a realistic manner. Despite the “automation” of these computer-aided drawings, the realism or correct schematization of microscopic organisms still depends on the skills of the authors, including their artistic concerns.
References
- Almeida, D. & Gusmão, L. (2015) Modification to the Barber and Keane’s method of illustrating fungi. Mycosphere, 6, 630–633. https://doi.org/10.5943/mycosphere/6/5/12
- Barber, P.A. & Keane, P.J. (2007) A novel method of illustrating microfungi. Fungal Diversity, 27 (1), 1–10.
- Bober, S. & Riehl, T. (2014) Adding depth to line artwork by digital stippling—a step-by-step guide to the method. Organisms Diversity & Evolution, 14, 327–337. https://doi.org/10.1007/s13127-014-0173-7
- Coleman, C.O. (2018) Shadings in digital taxonomic drawings. Zoosystematics and Evolution, 94 (2), 529–533. https://doi.org/10.3897/zse.94.28624
- Coleman, C.O. (2003) “Digital inking”: How to make perfect line drawings on computers. Organisms Diversity & Evolution, 14, 1–14. https://doi.org/10.1078/1439-6092-00081
- Coleman, C.O. (2006) Substituting time-consuming pencil drawings in arthropod taxonomy using stacks of digital photographs. Zootaxa, 1360 (1), 61–68. https://doi.org/10.11646/zootaxa.1360.1.4
- Coleman, C.O. (2009) Drawing setae, the digital way. Zoosystematics and Evolution, 85 (2), 305–310. https://doi.org/10.1002/zoos.200900008
- Fisher, J.R., Dowling, A.P. & Fisher, J.R. (2010) Modern methods and technology for doing classical taxonomy. Acarologia, 50 (3), 395–409. https://doi.org/10.1051/acarologia/20101981
- Holzenthal, R.W. (2008) Digital illustration of insects. American Entomologist, 54 (4), 218–221. https://doi.org/10.1093/ae/54.4.218
- Isbert, W., Carrassón, M., Pérez-del-Olmo, A. & Montero, F.E. (2017) A new species of Tinrovia Mamaev, 1987 (Monogenea: Microcotylidae) from the deep-sea fish Notacanthus bonaparte Risso (Notacanthiformes: Notacanthidae) in the Western Mediterranean and the North East Atlantic. Systematic Parasitology, 94 (5), 609–619. https://doi.org/10.1007/s11230-017-9727-3
- Montesanto, G. (2016) Drawing setae: A GNU way for digital scientific illustrations. Nauplius, 24, 1–6. https://doi.org/10.1590/2358-2936e2016017
- Pérez-del-Olmo, A., Dallarés, S., Georgieva, S., Constenla, M., Kostadinova, A. & Carrassón, M. (2019) Species of Lepidapedon Stafford, 1904 (Digenea: Lepidapedidae) from deep-sea fishes in the Western Mediterranean: molecular and morphological evidence. Systematic Parasitology, 96 (2), 149–169. https://doi.org/10.1007/s11230-019-09845-z
- Price, E.W. (1943) North American monogenetic trematodes: VI. The family Diclidophoridae (Diclidophoroidea). Journal of the Washington Academy of Sciences, 33 (2), 44–54. [http://www.jstor.org/stable/24531863]
- Sánchez-García, N., Padrós, F., Raga, J.A. & Montero, F.E. (2011) Comparative study of the three attachment mechanisms of diplectanid monogeneans. Aquaculture, 318 (3–4), 290–299. https://doi.org/10.1016/j.aquaculture.2011.05.021
- van Beneden, P.J. & Hesse, C.E. (1863) Recherches sur les Bdellodes (Hirudinées) et les Trématodes marins. Mémoires de l’Academie royale de Belgique, 34, 1–150. https://doi.org/10.5962/bhl.title.11767
- Yamaguti, S. (1963) Systema Helminthum. Monogenea and Aspidocotylea. Vol. IV. Interscience Publishers, Wiley, New York, New York, 699 pp.