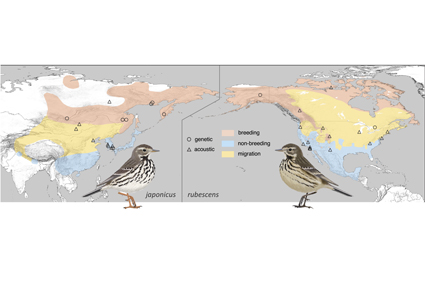
Abstract
The Buff-bellied Pipit Anthus rubescens comprises two allopatric subspecies groups: A. r. rubescens and A. r. alticola in North America and A. [r.] japonicus in north-east Asia. Despite their great morphological resemblance in breeding plumage, most individuals can be assigned to one or the other subspecies group in non-breeding plumage. Allopatric distributions, morphological differentiation and previously reported molecular divergence suggested the need for additional taxonomic study to assess the rank of these two populations. To resolve the taxonomy of the Buff-bellied Pipit species complex we analysed i) two mitochondrial DNA (mtDNA) loci and ii) nine bioacoustic parameters across 69 sound recordings (338 flight calls) recovered from public databases using principal component analysis and Euclidean distance measures. By comparing our mtDNA and call divergence measures with similar values measured between long-recognised species pairs of the genus, we show that the level of mitochondrial and acoustic divergence between the two Buff-bellied Pipit subspecies groups is typical of species-level divergence in the genus Anthus. Therefore, we recommend splitting the Buff-bellied Pipit species complex into two species: Anthus rubescens (American Pipit) and Anthus japonicus (Siberian Pipit). Our results also suggest that the Water Pipit A. spinoletta deserves taxonomic reassessment as its lineages are highly divergent in acoustics and mtDNA, while mtDNA relationships suggest paraphyly relative to the Rock Pipit A. petrosus. Our work highlights the crucial importance of integrative approaches in taxonomy and the usefulness of bioacoustics in studying cryptic diversity.
References
- Alström, P. & Mild, K. (1996) The identification of Rock, Water and Buff-bellied pipits. Alula, 4, 161–175.
- Alström, P. & Mild, K. (2003) Pipits and Wagtails of Europe, Asia and North America. Christopher Helm, London and Princeton University Press, Princeton, New Jersey, 496 pp.
- Alström, P. & Ranft, R. (2003) The use of sounds in avian systematics and the importance of bird sound archives. Bulletin of the British Ornithologists’ Club, 123, 114–135.
- Alström, P. & Ödeen, A. (2002) Incongruence between mitochondrial DNA, nuclear DNA and non-molecular data in the avian genus Motacilla: implications for estimates of species phylogenies. In: Alström, P., Species Limits and Systematics in Some Passerine Birds. PhD Thesis, Uppsala University, Uppsala, pp. 2–18.
- Alström, P., Olsson, U., Rasmussen, P.C., Yao, C.-T., Ericson, P.G.P. & Sundberg, P. (2007) Morphological, vocal and genetic divergence in the Cettia acanthizoides complex (Aves: Cettiidae). Zoological Journal of the Linnean Society, 149, 437–452. https://doi.org/10.1111/j.1096-3642.2007.00250.x
- Alström, P., Rasmussen, P.C., Olsson, U. & Sundberg, P. (2008) Species delimitation based on multiple criteria: the Spotted Bush Warbler Bradypterus thoracicus complex (Aves, Megaluridae). Zoological Journal of the Linnean Society, 154, 291–307. https://doi.org/10.1111/j.1096-3642.2008.00418.x
- Alström, P., Xia, C., Rasmussen, P.C., Olsson, U., Dai, B., Zhao, J., Leader, P.J., Carey, G.J., Dong, L., Cai, T., Holt, P.I., Le Manh, H., Song, G., Liu, Y., Zhang, Y. & Lei, F. (2015a) Integrative taxonomy of the Russet Bush Warbler Locustella mandelli complex reveals a new species from central China. Avian Research, 6, 9. https://doi.org/10.1186/s40657-015-0016-z
- Alström, P., Jønsson, K., Fjeldså, J., Ödeen, A., Ericson, P.G.P. & Irestedt, M. (2015b) Dramatic niche shifts and morphological change in two insular bird species. Royal Society Open Science 2: 140364. https://doi.org/10.1098/rsos.140364
- Alström, P., Rasmussen, P.C., Zhao, C., Xu, J., Dalvi, S., Cai, T., Guan, Y., Zhang, R., Kalyakin, M.V., Lei, F. & Olsson, U. (2016) Integrative taxonomy of the Plain-backed Thrush (Zoothera mollissima) complex (Aves, Turdidae) reveals cryptic species, including a new species. Avian Research, 7, 1. https://doi.org/10.1186/s40657-016-0037-2
- Alström, P., Rasmussen, P.C., Xia, C., Zhang, L., Liu, C., Magnusson, J., Shafaeipour, A. & Olsson, U. (2021a) Morphology, vocalizations, and mitochondrial DNA suggest that the Graceful Prinia is two species. Ornithology, 138 (2), ukab014. https://doi.org/10.1093/ornithology/ukab014
- Alström, P., van Linschooten, J., Donald, P.F., Sundev, G., Mohammadi, Z., Ghorbani, F., Shafaeipour, A., van den Berg, A., Robb, M., Aliabadian, M., Wei, C., Lei, F., Oxelman, B. & Olsson, U. (2021b) Multiple species delimitation approaches applied to the avian lark genus Alaudala. Molecular Phylogenetics and Evolution, 154, 106994. https://doi.org/10.1016/j.ympev.2020.106994
- Bairlein, F., Norris, D.R., Nagel, R., Bulte, M., Voigt, C.C., Fox, J.W., Hussel, D.J.T. & Schmaljohann, H. (2012) Cross-hemisphere migration of a 25 g songbird. Biology Letters, 8 (4), 505–507. https://doi.org/10.1098/rsbl.2011.1223
- Battey, C.J. & Klicka, J. (2017) Cryptic speciation and gene flow in a migratory songbird species complex: Insights from the Red-eyed Vireo (Vireo olivaceus). Molecular Phylogenetics and Evolution, 113, 67–75. https://doi.org/10.1016/j.ympev.2017.05.006
- BirdLife International (2022) Species factsheet: Anthus rubescens. Available from: http://datazone.birdlife.org/species/factsheet/buff-bellied-pipit-anthus-rubescens (accessed 25 July 2022)
- Center for Conservation Bioacoustics (2016) Raven Lite: Interactive Sound Analysis Software (Version 2.0.1). Cornell Lab of Ornithology, Ithaca, New York, Computer Software. Available from: http://ravensoundsoftware.com/ (accessed 18 July 2023)
- Clements, J.F. (2007) Clements Checklist of Birds of the World. Comstock Publishing Associates/Cornell University Press, Ithaca, New York, 864 pp.
- Clements, J.F., Schulenberg, T.S., Iliff, M.J., Fredericks, T.A., Gerbracht, J.A., Lepage, D., Billerman, S.M., Sullivan, B.L. & Wood, C.L. (2022) The eBird/Clements Checklist of Birds of the World. Version 2022. Available from: https://www.birds.cornell.edu/clementschecklist/download/ (accessed 30 January 2023)
- Collinson, J.M., Dufour, P., Hamza, A.A., Lawrie, Y., Elliott, M., Barlow, C. & Crochet, P. A. (2017) When morphology is not reflected by molecular phylogeny: the case of three ‘orange-billed terns’ Thalasseus maximus, Thalasseus bergii and Thalasseus bengalensis (Charadriiformes: Laridae). Biological Journal of the Linnean Society, 121 (2), 439–445. https://doi.org/10.1093/biolinnean/blw049
- Cramp, S. & Simmons, K.E.L. (1983) The Birds of the Western Palearctic. Vol. V. Oxford University Press, Oxford, 1136 pp.
- Delmore, K.E. & Irwin, D.E. (2014) Hybrid songbirds employ intermediate routes in a migratory divide. Ecology Letters, 17 (10), 1211–1218. https://doi.org/10.1111/ele.12326
- de Queiroz, K. (2007) Species concepts and species delimitation. Systematic Biology, 56(6), 879–886. https://doi.org/10.1080/10635150701701083
- Dor, R., Safran, R.J., Sheldon, F.H., Winkler, D.W. & Lovette, I.J. (2010) Phylogeny of the genus Hirundo and the Barn Swallow subspecies complex. Molecular Phylogenetics and Evolution, 56 (1), 409–418. https://doi.org/10.1016/j.ympev.2010.02.008
- Dufresnes, C. & Jablonski, D. (2022) A genomics revolution in amphibian taxonomy. Science, 377 (6612), 1272–1272. https://doi.org/10.1126/science.ade5002
- Dray, S. & Dufour, A.-B. (2007) The ade4 Package: implementing the duality diagram for ecologists. Journal of Statistical Software, 22 (4), 1–20. https://doi.org/10.18637/jss.v022.i04
- Drovetski, S.V., Reeves, A.B., Red’kin, Y.A., Fadeev, I.V., Koblik, E.A., Sotnikov, V.N. & Voelker, G. (2018) Multi-locus reassessment of a striking discord between mtDNA gene trees and taxonomy across two congeneric species complexes. Molecular Phylogenetics and Evolution, 120, 43–52. https://doi.org/10.1016/j.ympev.2017.11.023
- Farnsworth, A. & Lovette, I.J. (2008) Phylogenetic and ecological effects on interspecific variation in structurally simple avian vocalizations. Biological Journal of the Linnean Society, 94 (1), 155–173. https://doi.org/10.1111/j.1095-8312.2008.00973.x
- Fijen, T.P. (2014) Flight call identification of Rock Pipit and Water Pipit. Dutch Birding, 36, 87–95.
- Garner, M., Perlman, Y., Kiat, Y. & Collinson, J.M. (2015) Water pipits: Three species rather than one? British Birds, 108 (1), 42–48.
- Gill, F., Donsker, D. & Rasmussen, P. (Eds.) (2023) IOC World Bird List. Version 13.1. Available from: https://www.worldbirdnames.org/new/ (accessed 18 July 2023)
- Gwee, C.Y., Eaton, J.A., Garg, K.M., Alström, P., Van Balen, S., Hutchinson, R.O., Prawiradilaga, D.M., Le, M.H. & Rheindt, F.E. (2019) Cryptic diversity in Cyornis (Aves: Muscicapidae) jungle-flycatchers flagged by simple bioacoustic approaches. Zoological Journal of the Linnean Society, 186 (3), 725–741. https://doi.org/10.1093/zoolinnean/zlz003
- Harris, R.B., Alström, P., Ödeen, A. & Leaché, A.D. (2018) Discordance between genomic divergence and phenotypic variation in a rapidly evolving avian genus (Motacilla). Molecular Phylogenetics and Evolution, 120, 183–195. https://doi.org/10.1016/j.ympev.2017.11.020
- Helbig, A.J., Knox, A.G., Parkin, D.T., Sangster, G. & Collinson, M. (2002) Guidelines for assigning species rank. Ibis, 144 (3), 518–525. https://doi.org/10.1046/j.1474-919X.2002.00091.x
- Hopkins, D.M. (1959) Cenozoic history of the Bering land bridge. Science, 129 (3362), 1519–1528. https://doi.org/10.1126/science.129.3362.1519
- Irwin, D.E., Irwin, J.H., Greenberg, R. & Marra, P.P. (2005) Siberian migratory divides: the role of seasonal migration in speciation. In: Greenberg, R. & Marra, P.P. (Eds.), Birds of Two Worlds: the Ecology and Evolution of Migration. Johns Hopkins University Press, Baltimore, Maryland, pp. 27–40.
- Hendricks, P. & Verbeek, N.A. (2020) American Pipit (Anthus rubescens). Version 1.0. In: Billerman, S.M. (Ed.), Birds of the World. Cornell Lab of Ornithology, Ithaca, New York. [program] https://doi.org/10.2173/bow.amepip.01
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Lee, C.-T. & Birch, A. (2002) Notes on the distribution, vagrancy, and field identification of American Pipit and “Siberian Pipit”. North American Birds, 56, 389–398.
- Mallet, J. (2008) Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Philosophical Transactions of the Royal Society B: Biological Sciences, 363 (1506), 2971–2986. https://doi.org/10.1098/rstb.2008.0081
- Marler, P. (2004) Bird calls: their potential for behavioral neurobiology. Annals of the New York Academy of Sciences, 1016, 31–44. https://doi.org/10.1196/annals.1298.034
- Martin, R. (2013) AVESRARES, Identification of Olive-backed and Tree Pipit by call. Available from: https://avesrares.wordpress.com/2013/09/27/identification-of-olive-backed-and-tree-pipit-by-call/ (accessed 30 January 2023)
- Mayr, R. (1942) Systematics and the Origin of Species. Harvard University Press, Cambridge, Massachusetts, 372 pp.
- McLaughlin, J.F., Faircloth, B.C., Glenn, T.C. & Winker, K. (2020) Divergence, gene flow, and speciation in eight lineages of trans‐Beringian birds. Molecular Ecology, 29 (18), 3526–3542. https://doi.org/10.1111/mec.15574
- Nabholz, B., Lanfear, R. & Fuchs, J. (2016) Body mass‐corrected molecular rate for bird mitochondrial DNA. Molecular Ecology, 25 (18), 4438–4449. https://doi.org/10.1111/mec.13780
- Ng, E.Y., Eaton, J.A., Verbelen, P., Hutchinson, R.O. & Rheindt, F.E. (2016) Using bioacoustic data to test species limits in an Indo-Pacific Island radiation of Macropygia cuckoo doves. Biological Journal of the Linnean Society, 118 (4), 786–812. https://doi.org/10.1111/bij.12768
- Oksanen, J., Blanchet, F.J., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Henry, M., Stevens, H., Szoecs, E. & Wagner, H. (2019) vegan: Community Ecology Package. R Package Version 2.5-6. Available from: https://CRAN.R-project.org/package=vegan (accessed 18 July 2023)
- Päckert, M., Martens, J., Sun, Y.H. & Veith, M. (2004) The radiation of the Seicercus burkii complex and its congeners (Aves: Sylviidae): molecular genetics and bioacoustics. Organisms Diversity & Evolution, 4 (4), 341–364. https://doi.org/10.1016/j.ode.2004.06.002
- Päckert, M. (2018) Song: The learned language of three major bird clades. In: Tietze, D. (Ed.), Bird Species. Springer, Cham, pp. 75–94. https://doi.org/10.1007/978-3-319-91689-7_5
- Padial, J.M., Miralles, A., De la Riva, I. & Vences, M. (2010) The integrative future of taxonomy. Frontiers in Zoology, 7 (1), 1–14. https://doi.org/10.1186/1742-9994-7-16
- Pavia, M., Drovetski, S.V., Boano, G., Conway, K.W., Pellegrino, I. & Voelker, G. (2021) Elevation of two subspecies of Dunnock Prunella modularis to species rank. Bulletin of the British Ornithologists’ Club, 141 (2), 199–210. https://doi.org/10.25226/bboc.v141i2.2021.a10
- Pavlova, A., Zink, R.M., Drovetski, S.V., Red’kin, Y. & Rohwer, S. (2003) Phylogeographic patterns in Motacilla flava and Motacilla citreola: species limits and population history. The Auk, 120 (3), 744–758. https://doi.org/10.1093/auk/120.3.744
- Pertierra, L.R., Segovia, N.I., Noll, D., Martinez, P.A., Pliscoff, P., Barbosa, A., Aragón, P., Raya Rey, A., Pistorius, P., Trathan, P., Polanowski, A., Bonadonna, F., Le Bohec, C., Bi, K., Whang-Claypool, C.Y., González-Acuña, D., Dantas, G.P.M, Bowie, R.C.K., Poulin, E. & Vianna, J.A. (2020) Cryptic speciation in gentoo penguins is driven by geographic isolation and regional marine conditions: Unforeseen vulnerabilities to global change. Diversity and Distributions, 26 (8), 958–975. https://doi.org/10.1111/ddi.13072
- Peters, J.L., McCracken, K.G., Pruett, C.L., Rohwer, S., Drovetski, S.V., Zhuravlev, Y.N., Kulikova, I., Gibson, D.D. & Winker, K. (2012) A parapatric propensity for breeding precludes the completion of speciation in Common Teal (Anas crecca, sensu lato). Molecular Ecology, 21 (18), 4563–4577. https://doi.org/10.1111/j.1365-294X.2012.05711.x
- Pietersen, D.W., McKechnie, A.E., Jansen, R., Little, I.T. & Bastos, A.D. (2019) Multi‐locus phylogeny of African pipits and longclaws (Aves: Motacillidae) highlights taxonomic inconsistencies. Ibis, 161 (4), 781–792. https://doi.org/10.1111/ibi.12683
- Porter, R. & Aspinall, S. (2013) Birds of the Middle East. Bloomsbury Publishing, London, 400 pp.
- Porter, C.K. & Benkman, C.W. (2019) Character displacement of a learned behaviour and its implications for ecological speciation. Proceedings of the Royal Society B, 286 (1908), 20190761. https://doi.org/10.1098/rspb.2019.0761
- Potvin, D.A., Parris, K.M. & Mulder, R.A. (2013) Limited genetic differentiation between acoustically divergent populations of urban and rural silvereyes (Zosterops lateralis). Evolutionary Ecology, 27 (2), 381–391. https://doi.org/10.1007/s10682-012-9591-1
- Price, T. (2008) Speciation in Birds. Roberts & Co., Greenwood Village, Colorado, 470 pp.
- Reeves, A.B., Drovetski, S.V. & Fadeev, I.V. (2008) Mitochondrial DNA data imply a stepping‐stone colonization of Beringia by Arctic Warbler Phylloscopus borealis. Journal of Avian Biology, 39 (5), 567–575. https://doi.org/10.1111/j.0908-8857.2008.04421.x
- Rubinoff, D. & Holland, B.S. (2005) Between two extremes: mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference. Systematic Biology, 54 (6), 952–961. https://doi.org/10.1080/10635150500234674
- Safran, R.J., Scordato, E.S.C., Wilkins, M.R., Hubbard, J.K., Jenkins, B.R., Albrecht, T., Flaxman, S.M., Karaardiç, H., Vortman, Y., Lotem, A., Nosil, P., Pap, P., Shen, S., Chan, S.-F., Parchman, T.L. & Kane, N.C. (2016) Genome‐wide differentiation in closely related populations: the roles of selection and geographic isolation. Molecular Ecology, 25 (16), 3865–3883. https://doi.org/10.1111/mec.13740
- Saitoh, T., Alström, P., Nishiumi, I., Shigeta, Y., Williams, D., Olsson, U. & Ueda, K. (2010) Old divergences in a boreal bird supports long-term survival through the Ice Ages. BMC Evolutionary Biology, 10 (1), 1–13. https://doi.org/10.1186/1471-2148-10-35
- Sangster, G. (2022) The taxonomic status of Palearctic and Nearctic populations of Northern Goshawk Accipiter gentilis (Aves, Accipitridae): New evidence from vocalisations. Vertebrate Zoology, 72, 445–456. https://doi.org/10.3897/vz.72.e85419
- Shirihai, H. & Svensson, L. (2018) Handbook of Western Palearctic Birds. Vol. 1. Passerines: Larks to Warblers. Bloomsbury Publishing, London, 3312 pp.
- Sin, Y.C.K., Eaton, J.A., Hutchinson, R.O. & Rheindt, F.E. (2022) Re-assessing species limits in a morphologically cryptic Australasian kingfisher lineage (Coraciiformes: Halcyonidae) using bioacoustic data. Biological Journal of the Linnean Society, 136 (4), 489–505. https://doi.org/10.1093/biolinnean/blac047
- Song, G., Zhang, R., Alström, P., Irestedt, M., Cai, T., Qu, Y., Ericson, P.G.P., Fjeldså, J. & Lei, F. (2018) Complete taxon sampling of the avian genus Pica (magpies) reveals ancient relictual populations and synchronous Late‐Pleistocene demographic expansion across the Northern Hemisphere. Journal of Avian Biology, 49 (2), jav-01612. https://doi.org/10.1111/jav.01612
- Speybroeck, J., Beukema, W., Dufresnes, C., Fritz, U., Jablonski, D., Lymberakis, P., Martínez-Solano, I., Razzetti, E., Vamberger, M., Vences, M., Vörös, J. & Crochet, P.A. (2020) Species list of the European herpetofauna—2020 update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphibia-Reptilia, 41 (2), 139–189. https://doi.org/10.1163/15685381-bja10010
- Tan, H.Z., Ng, E.Y.X., Tang, Q., Allport, G.A., Jansen, J.J., Tomkovich, P.S. & Rheindt, F.E. (2019) Population genomics of two congeneric Palaearctic shorebirds reveals differential impacts of Quaternary climate oscillations across habitats types. Scientific Reports, 9 (1), 1–9. https://doi.org/10.1038/s41598-019-54715-9
- Turbek, S.P., Schield, D.R., Scordato, E.S., Contina, A., Da, X.W., Liu, Y., Liu, Y., Pagani- Núñez, E., Ren, Q.-M., Smith, C.C.R., Stricker, C.A., Wunder, M., Zonana, D.M. & Safran, R.J. (2022) A migratory divide spanning two continents is associated with genomic and ecological divergence. Evolution, 76 (4), 722–736. https://doi.org/10.1111/evo.14448
- van Els, P. & Norambuena, H.V. (2018) A revision of species limits in Neotropical pipits Anthus based on multilocus genetic and vocal data. Ibis, 160 (1), 158–172. https://doi.org/10.1111/ibi.12511
- Winker, K., McCracken, K.G., Gibson, D.D. & Peters, J.L. (2013) Heteropatric speciation in a duck, Anas crecca. Molecular Ecology, 22 (23), 5922–5935. https://doi.org/10.1111/mec.12525
- Zink, R.M., Rohwer, S., Andreev, A.V. & Dittmann, D.L. (1995) Trans-Beringia comparisons of mitochondrial DNA differentiation in birds. The Condor, 97 (3), 639–649. https://doi.org/10.2307/1369173
- Zink, R.M., Rohwer, S., Drovetski, S., Blackwell-Rago, R.C. & Farrell, S.L. (2002) Holarctic phylogeography and species limits of three-toed woodpeckers. Condor, 104 (1), 167–170. https://doi.org/10.1093/condor/104.1.167
- Zink, R.M. & Barrowclough, G.F. (2008) Mitochondrial DNA under siege in avian phylogeography. Molecular Ecology, 17 (9), 2107–2121. https://doi.org/10.1111/j.1365-294X.2008.03737.x